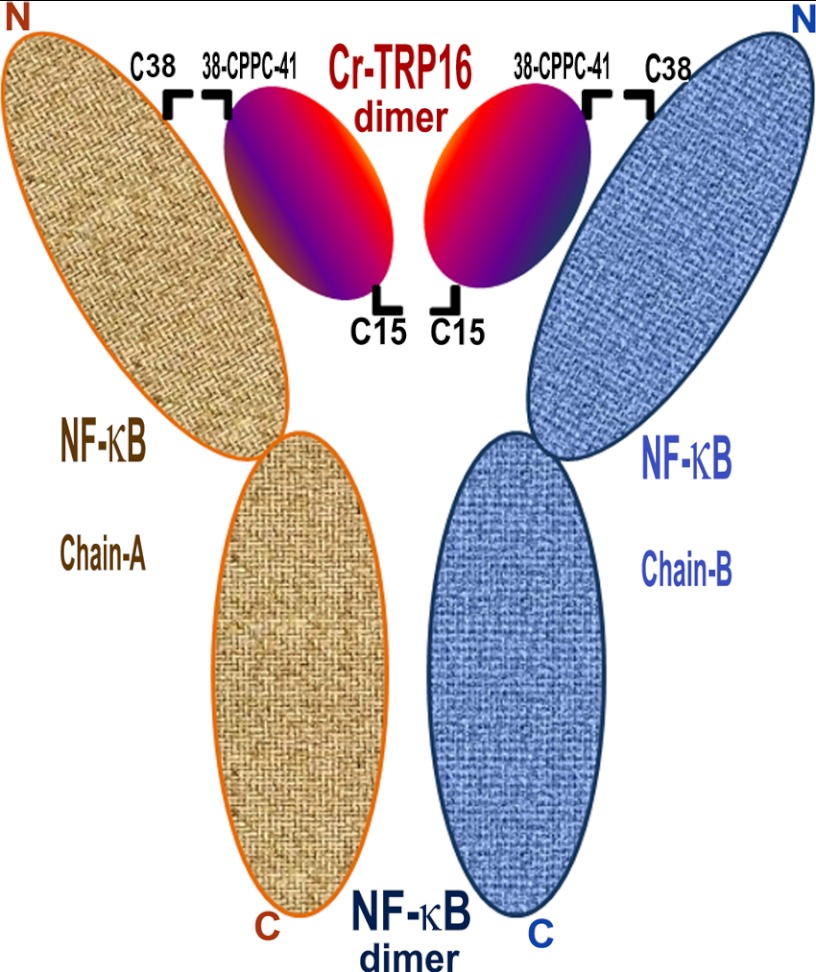
FIGURE 8.
Model for the interaction of Cr-TRP16 dimer with NF-κB dimer. Based on the previously published human NF-κB p50 homodimer-DNA complex and human Trx-NF-κB p50 peptide complex structures and our structural, biophysical, and biochemical results, we propose that Cr-TRP16 forms a homodimer by making a disulfide link through Cys-15 and is likely to occupy the location where DNA binds to NF-κB. The active site motif WCPPC of Cr-TRP16 (through Cys-38 and Cys-41) is likely to interact with the redox active Cys-62 of NF-κB dimer. For the ease of residue numbering, a p50 homodimer is assumed for NF-κB, and the C-terminal cysteines of NF-κB that promote dimerization of NF-κB are not shown for simplicity.