Abstract
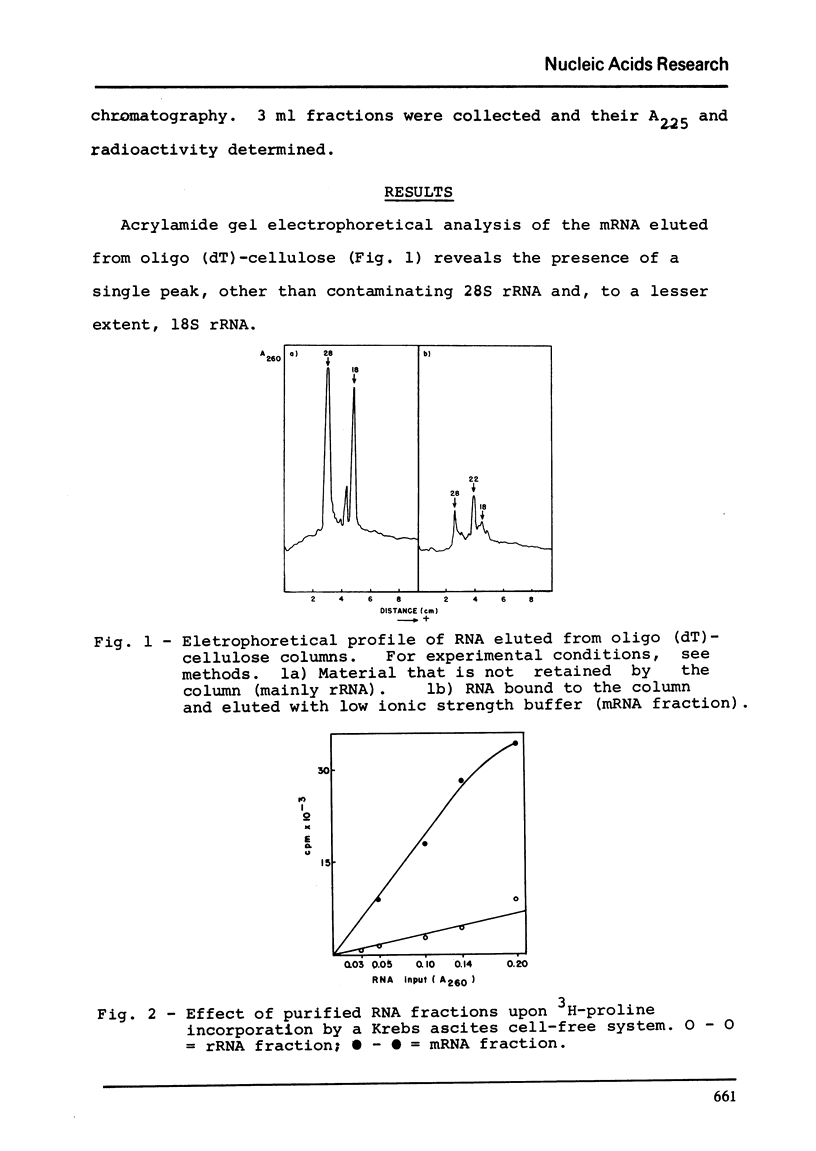
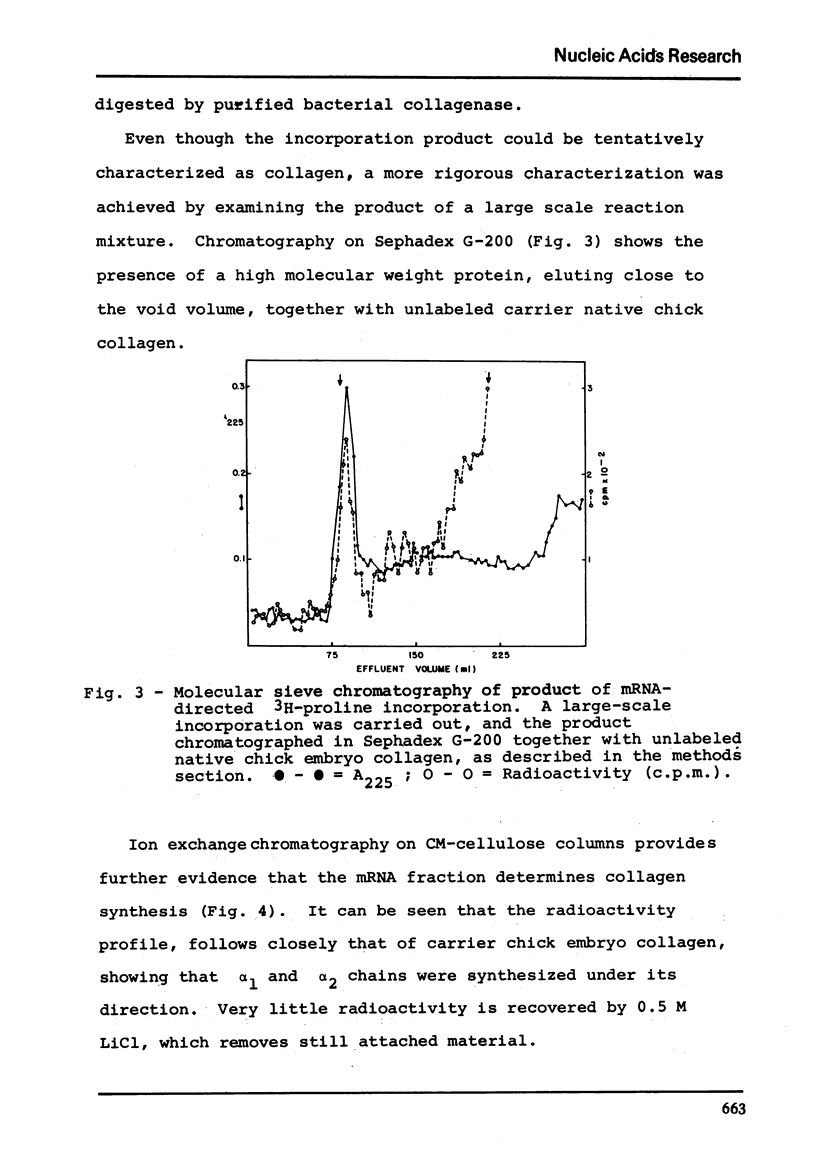
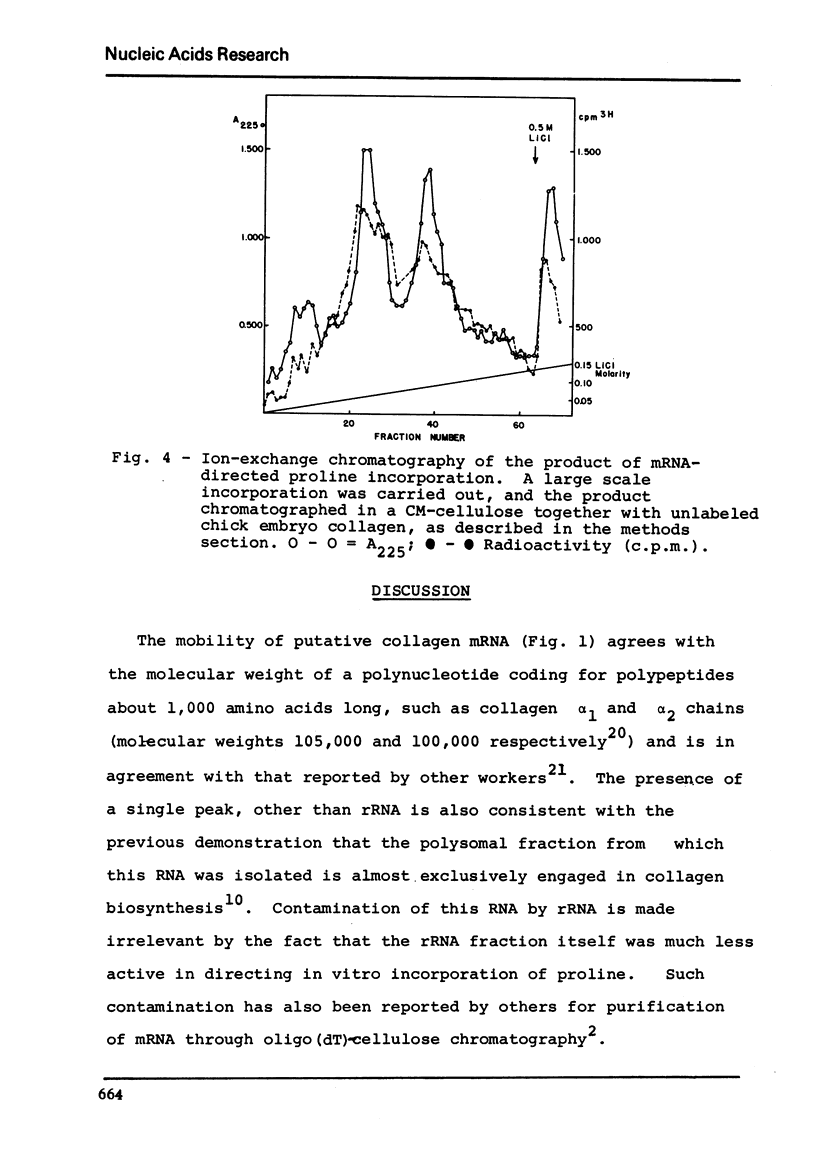
Chick embryo collagen-synthesizing polysomes were isolated by differential centrifugation. RNA extracted from these particles was chromatographed in oligo(dT)-cellulose solumns and the mRNA thus obtained characterized as collagen mRNA by its electrophoetical mobility in acrylamide gels (equivalent to 1.05 x 10-6 daltons) and its effect upon a cell-free system derived from Krebs ascites tumor cells. The incorporation of 3H-proline was markedly dependent upon rabbit reticulocyte initiation factors and inhibited by initiation inhibitors such as aurintricaboxilate and pyrocatechol violet. The incorporation product was characterized as collagen by its lack of tryptophan, digestibility by purified bacterial collagenase, and by its co-chromatography with unlabled chick collagen in Sephadex G-200 and CM-cellulose columns.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Connolly A. D., Grant M. E., Jackson D. S. Presumptive mRNA for procollagen: occurrence in membrane bound ribosomes of embryonic chick tendon fibroblasts. FEBS Lett. 1974 Apr 15;41(1):85–88. doi: 10.1016/0014-5793(74)80960-9. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Grollman A. P. Pyrocatechol violet: an inhibitor of initiation of protein synthesis. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1049–1059. doi: 10.1016/0006-291x(73)90571-8. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A., Parry D. A., Piez K. A., Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol. 1973 Sep 5;79(1):137–148. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- KRETSINGER R. H., MANNER G., GOULD B. S., RICH A. SYNTHESIS OF COLLAGEN ON POLYRIBOSOMES. Nature. 1964 May 2;202:438–441. doi: 10.1038/202438a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lukens L. N. Collagen synthesis on polysomes in vivo and in vitro. Nat New Biol. 1971 Jul 14;232(28):37–40. doi: 10.1038/newbio232037a0. [DOI] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques N., Wang L., Myiashita M., Stolf A. M., Balsamo J., Brentani R. Reiteration of DNA complementary to a cytoplasmic non-ribosomal RNA. Biochem Biophys Res Commun. 1973 Jul 2;53(1):317–325. doi: 10.1016/0006-291x(73)91436-8. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Mammalian messenger RNA. Essays Biochem. 1973;9:59–102. [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Metafora S., Terada M., Dow L. W., Marks P. A., Bank A. Increased efficiency of exogenous messenger RNA translation in a Krebs ascites cell lysate. Proc Natl Acad Sci U S A. 1972 May;69(5):1299–1303. doi: 10.1073/pnas.69.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington G. A., Kemp D. J., Rogers G. E. Isolation of feather keratin mRNA and its translation in a rabbit reticulocyte cell-free system. Nat New Biol. 1973 Nov 14;246(150):33–36. doi: 10.1038/newbio246033a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Persistent synthesis of 5S RNA when production of 28S and 18S ribosomal RNA is inhibited by low doses of actinomycin D. J Cell Physiol. 1968 Dec;72(3):235–246. doi: 10.1002/jcp.1040720311. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Synthesis of ovalbumin in a rabbit reticulocyte cell-free system programmed with hen oviduct ribonucleic acid. J Biol Chem. 1971 Dec 10;246(23):7407–7410. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]


