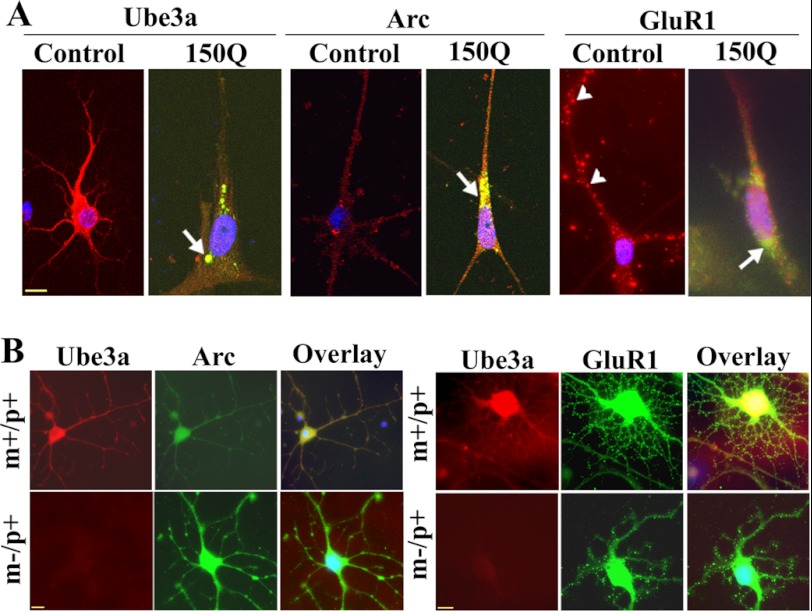
FIGURE 8.
Immunofluorescence staining of Ube3a, Arc, and GluR1 in primary cortical neuronal culture. A, primary cortical neurons (after 4 days of culture) were transiently transfected with tNhtt-150Q-EGFP constructs for 36 h and then subjected to immunofluorescence staining using antibodies against Ube3a, Arc, and GluR1. Rhodamine-conjugated secondary antibody was used to detect Ube3a, Arc, and GluR1. Nuclei were counterstained with DAPI. Arrows indicate the localization of Arc in the mutant huntingtin aggregates; arrowheads points to GluR1 puncta. Scale bar = 10 μm. The tNhtt-150Q-transfected cells showed significantly reduced numbers of neuronal processes compared with the control cells: control, 15.25 ± 3.6; and tNhtt-150Q, 4.64 ± 1.52 (p < 0.01). Neuronal branching of untransfected and transfected cells was calculated using NeuronStudio (Beta) software. At least nine cultured neurons in each group were used for analysis. B, double immunofluorescence staining of Arc/Ube3a and GluR1/Ube3a in primary cortical neuronal cultures (5 days) obtained from wild-type (m+/p+) and maternal Ube3a-deficient (m−/p+) mouse brains. Rhodamine-conjugated secondary antibody was used to detect Ube3a (mouse-specific), whereas FITC-conjugated secondary antibody was used to label Arc and GluR1 (rabbit-specific). Nuclei were counterstained with DAPI in overlaid images. Scale bars = 10 μm.