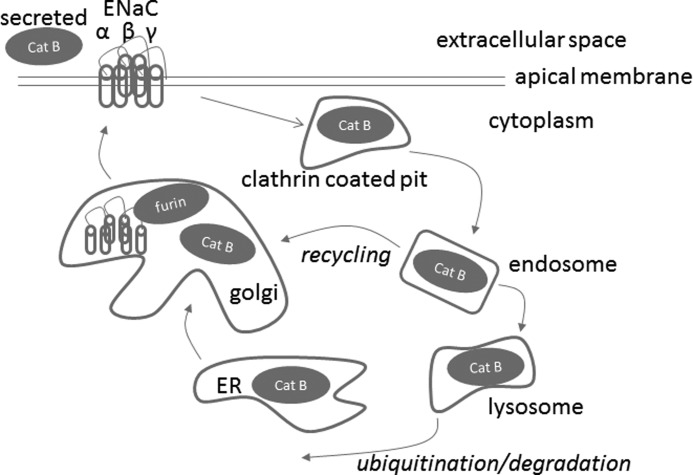
FIGURE 8.
Proposed model depicting the cathepsin B cleavage-dependent activation of ENaC. The cysteine protease cathepsin B (Cat B) is synthesized as a latent preproenzyme and post-translationally processed in the rough endoplasmic reticulum, Golgi, endosomal, and lysosomal compartments. Cathepsin B is secreted or found in the cytoplasm of certain epithelial cells. Unlike other cysteine proteases, cathepsin B exhibits both endopeptidase and exopeptidase activities. Cathepsin B is shown to potentially cleave the extracellular loop of the α subunit of ENaC either within the Golgi, extracellularly, or within endosomes.