FIGURE 3.
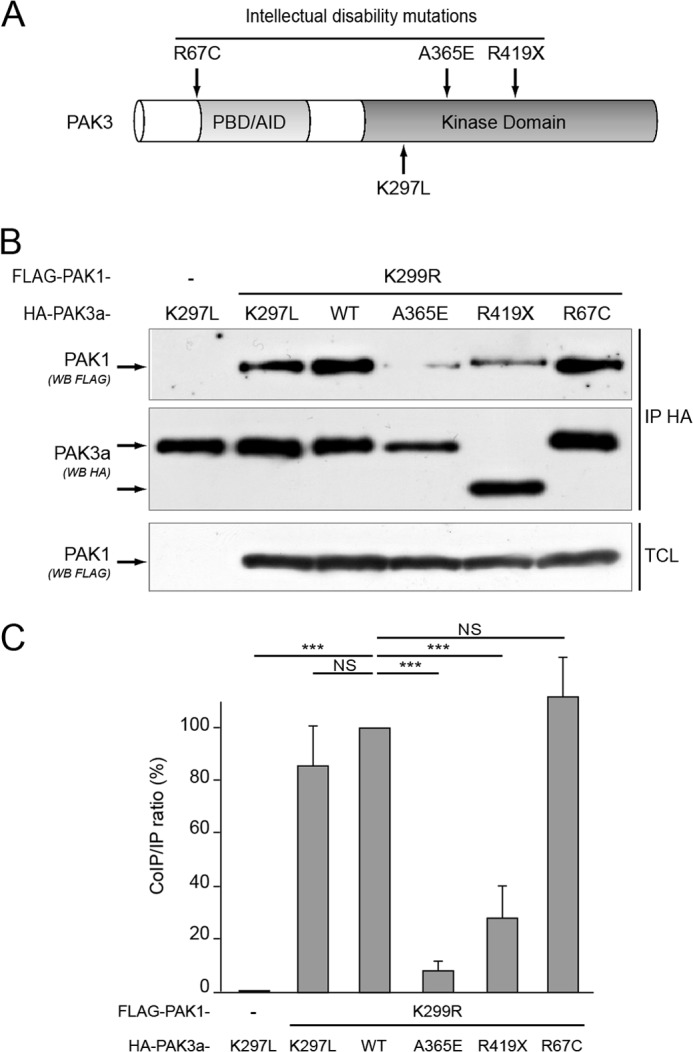
Two intellectual disability mutations of PAK3 affect their dimerization properties. A, location of three mutations responsible for intellectual disability. B, the A365E and R419X mutations affect the capacity to form heterodimers. HeLa cells were co-transfected with FLAG-PAK1-KD and HA-tagged PAK3a-KD, -WT, -A365E, -R419X, or -R67C. Co-immunoprecipitated proteins were revealed using FLAG antibodies (first panel) and HA-immunoprecipitated proteins (IP HA) were controlled by anti-HA labeling (second panel). Expression of FLAG PAK1 protein was controlled on TCL by an anti-FLAG Western blotting (third panel). Image shown is representative of three independent experiments. C, amount of FLAG-PAK1 proteins co-immunoprecipitated compared with the amount of HA-precipitated PAK proteins, in the experiment illustrated in B. Results are expressed relative to the PAK1/PAK3-WT interaction. Comparison with Student's t test: NS, p > 0.05; ***, p < 0.001, n = 3.
