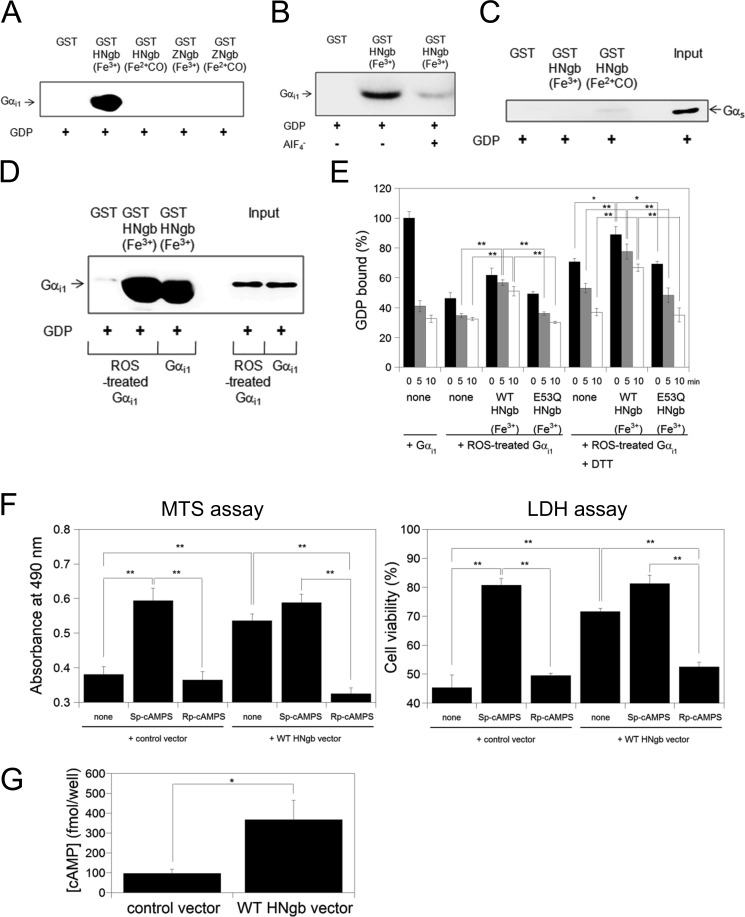
FIGURE 4.
Structural and Gαi1-binding properties of HNgb or ZNgb. A, GST pull-down assays with Ngb and rat myristoylated GDP-bound Gαi1. Western blot analyses were performed with anti-Gαi1 mouse monoclonal antibody. B, comparison of HNgb binding between different forms of recombinant human Gαi1. GDP-bound Gαi1 in the absence or presence of AlF4− was incubated with GST-HNgb or GST. C, GST pull-down assays with HNgb and Gαs. GDP-bound Gαs was incubated with GST-HNgb or GST, washed, and analyzed by Western blotting with anti-Gαs antibody. D, GST pull-down assays with HNgb and ROS-treated Gαi1. GST-HNgb or GST was incubated with untreated or ROS-treated human Gαi1. The same concentration and volume of untreated and ROS-treated human Gαi1 were used. E, effects of HNgb on dissociation of GDP from untreated or ROS-treated Gαi1. The amount of [3H]GDP bound to untreated Gαi1 in the absence of HNgb at 0 min was defined as 100%. Data are expressed as means ± S.E. from four independent experiments. *, p < 0.05, **, p < 0.01. F, protection by HNgb is mediated by cAMP. Differentiated SH-SY5Y cells were pretreated with Sp-cAMPS or Rp-cAMPS, and then incubated with hydrogen peroxide. Cell viability was measured by MTS and LDH assays. Each graph represents data from three independent experiments each carried out in triplicate. Data are expressed as means ± S.E. **, p < 0.01. G, WT HNgb increases intracellular cAMP concentration under oxidative stress. Differentiated SH-SY5Y cells were treated with hydrogen peroxide, and intracellular cAMP concentrations were determined by cAMP enzyme immunoassay kit. Data are expressed as means ± S.E. from five experiments. *, p < 0.05.