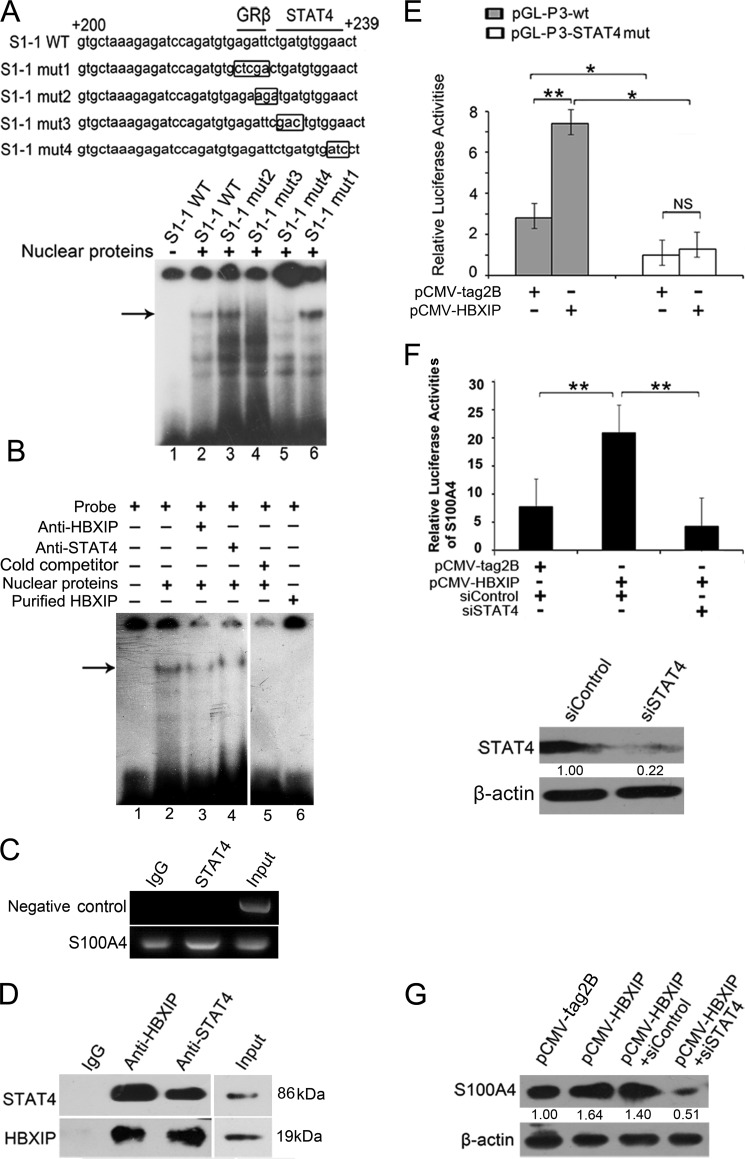
FIGURE 3.
HBXIP activates S100A4 via binding STAT4 involving the STAT4 element in +200/+239 region of S100A4 promote. A, interaction of nuclear proteins with mutants of +200/+239 region in S100A4 promoter was determined by EMSA. The mutated nucleotides were showed in box. B, binding of nuclear proteins to S1-1 fragment with the addition of anti-HBXIP or anti-STAT4 antibody was examined by EMSA. C, interaction of STAT4 with promoter region of S100A4 was examined by ChIP assay. The +208/+584 region of S100A4 promoter was amplified by PCR. The data presented are from three independent experiments. D, interaction between endogenous HBXIP and STAT4 was examined in MCF-7 cells by Co-IP assay. HBXIP and STAT4 in cell lysate were examined by Western blot, which was used as an input control. E, MCF-7 cells were transiently co-transfected with plasmids of pCMV-HBXIP (or pCMV-tag2B) and pGL-P3 wt (or pGL-P3-STAT4mut) constructs (*, p < 0.05, **, p < 0.01, versus control, Student's t test). The data presented are from three independent experiments; error bars represent standard error. F, MCF-7 cells were transiently co-transfected with plasmid pCMV-HBXIP (or pCMV-tag2B), 50 nm STAT4 siRNA (or control siRNA) and pGL-P3 constructs. Western blot showed the knockdown efficiency of 50 nm siRNAs for STAT4 in MCF-7 cells. (**, p < 0.01, versus control, Student's t test). The data presented are from three independent experiments; error bars represent standard error. G, MCF-7 cells were transiently transfected with pCMV-HBXIP (or pCMV-tag2B), pCMV-HBXIP, and 50 nm siSTAT4 (or siControl), respectively. The expression levels of S100A4 were examined by Western blot analysis.