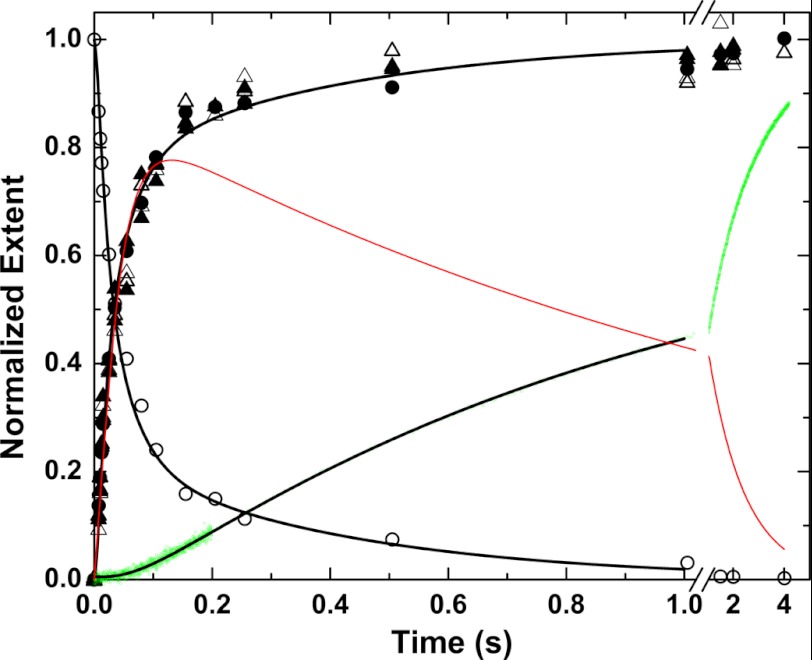
FIGURE 6.
Cleavage of IIQ271 by prothrombinase under conditions approximating single turnover. Kinetic measurements were performed following rapid mixing of equal volumes of 0.6 μm prothrombinase (0.6 μm Xa, 0.75 μm Va, and 300 μm PCPS) and 0.6 μm IIQ271 in the presence of 2 μm DAPA. Substrate depletion (○) and product formation (●) were measured discontinuously following rapid chemical quench and SDS-PAGE. Alternatively, proteinase product was inferred in the quenched samples by initial velocity measurements of S2238 hydrolysis (△) or by the enhanced steady state fluorescence of DAPA (▴). Product formation was also measured continuously by stopped flow (green dots) by monitoring the enhanced fluorescence of DAPA bound to the proteinase product. The black lines are drawn by simulation/fitting according to supplemental Scheme IS using the considerations, assumptions, and constants listed. The red line depicts the difference between the fitted product time course from rapid chemical quench and the fitted line for the continuous stopped-flow measurement.