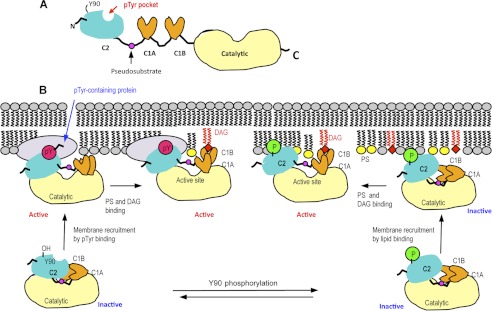
FIGURE 7.
Schematic domain structure of PKCθ (A) and a proposed mechanism of membrane binding and activation of PKCθ (B). A, domain organization of PKCθ is shown schematically with the location of the Tyr(P) (pTyr) binding pocket in the C2 domain indicated by a red arrow. B, in the resting state of PKCθ, Tyr90 is unphosphorylated, and neither the C2 nor C1B domain is fully exposed for optimal membrane interactions. In the canonical activation mechanism, phosphorylation of Tyr90 induces conformational changes that promote the membrane recruitment of PKCθ by lipid binding. Binding of the C2 domain to PS (yellow) and subsequent membrane penetration and DAG (red) binding of the C1B domain pull the pseudosubstrate region from the active site, resulting in enzyme activation. Regardless of Tyr90 phosphorylation, the C2 domain can bind Tyr(P) of a membrane-bound protein, which causes the activation of PKCθ through conformational changes that also lead to the removal of pseudosubstrate from the active site. PS and DAG binding may further activate the protein or prolong its membrane residence and/or activation.