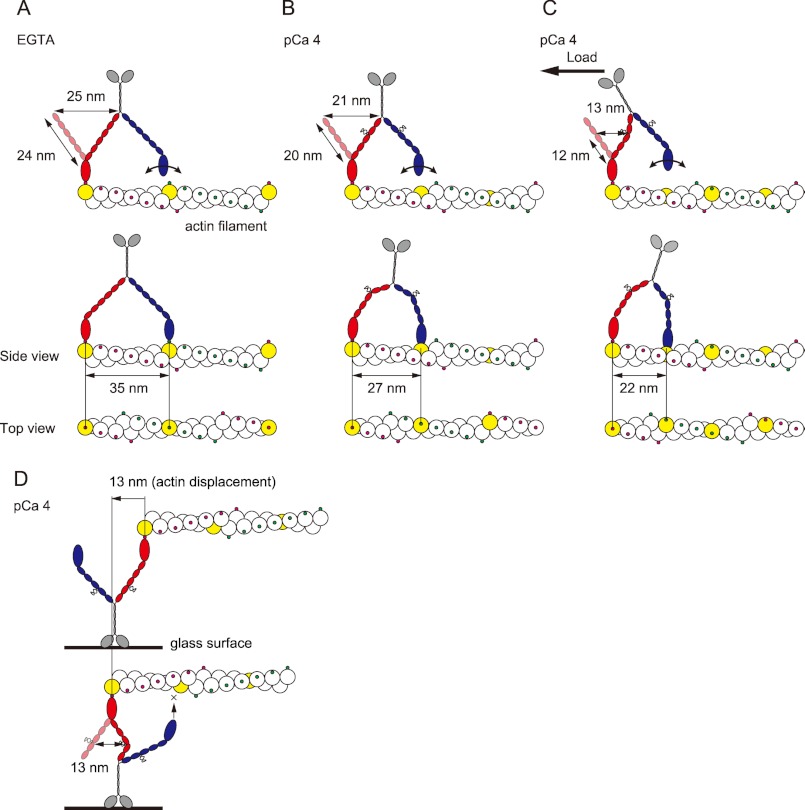
FIGURE 5.
Model of the stepwise movement of myosin XI after Ca2+-induced CaM dissociation along an actin filament. In each case, the lead head is colored red, and the trail head is blue. The putative actin subunit for binding estimated from step size is colored yellow. A, in EGTA, myosin XI generates 35-nm steps along the same azimuth on an actin filament with a long 27-nm neck. B, at pCa 4, the leading head cannot achieve the same azimuth relative to the actin filament because it has a short neck with four to five light chains, generating 27-nm steps, landing at a different azimuth about nine actin subunits forward. C, under the high load, the kink at the position in the fourth IQ motif due to the load causes the lever to act as if it has three light chains, generating 22-nm steps, landing about seven actin subunits forward. Myosin XI is able to achieve multiple steps, searching and then binding to the binding site at the upper side of the actin filament. D, in an in vitro motility assay, the diffusion of myosin is sterically restricted, especially on the z axis, which inhibits processive movement. The actin displacement is 13 nm, which is 37% of the 35 nm generated by intact myosin XI.