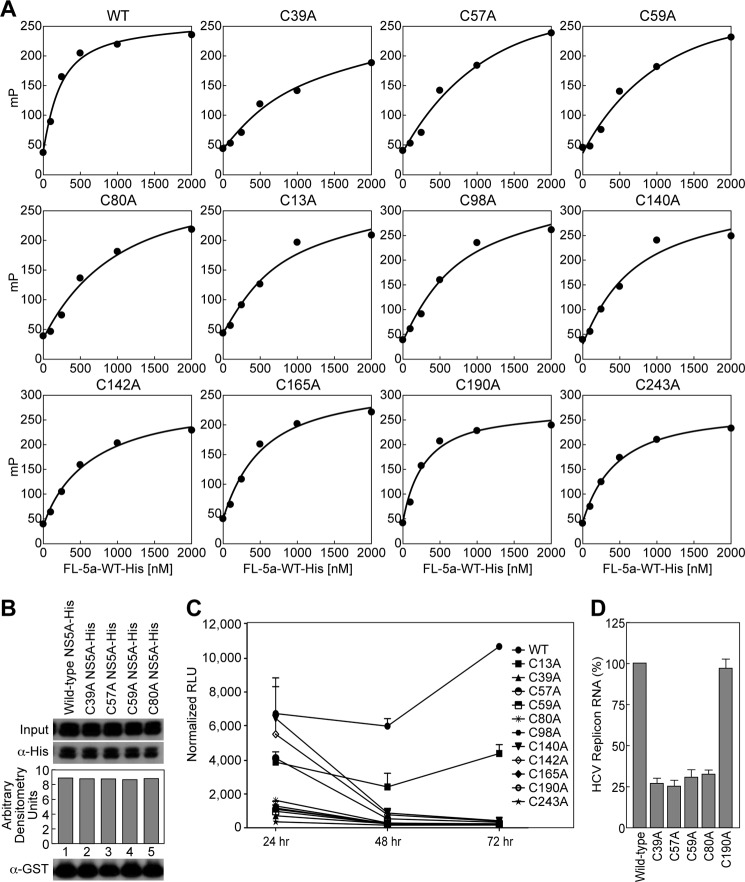
FIGURE 5.
Cysteines critical for dimerization are also important for RNA-binding and replication. A, NS5A (0–2000 nm) and 0.1 nm rU15-FI were gently mixed in binding reaction buffer (20 mm HEPES, pH 7.5, 100 mm NaCI, 5 mm MgCl2, and 0.5 mm TCEP) and incubated for 30 s at 25 °C. Binding of NS5A was measured by the change in polarization (mP). The data were fit to a hyperbola using KaleidaGraph (Synergy Software). B, full-length NS5A-His with alanine substitutions at Cys-39, Cys-57, Cys-59, and Cys-80 were tested for their ability to bind GST-NS5BΔ21 relative to wild-type NS5A. C, Huh7.5.1 cells were electroporated with luciferase reporter subgenomic RNA transcripts containing cysteine mutations in NS5A. The cells were lysed at the given time points and post-electroporation, and the replication fitness was measured using a luciferase assay. The results (triplicates) are representative of three independent experiments. D, Huh7-Con1 cells were transfected with wild-type or cysteine NS5A-His plasmids for 3 days. The cell lysates were incubated with Ni+ beads for 1 h, bound material was eluted, RNA was purified, and quantitative real time PCR was executed as described (18). The amount of HCV RNA precipitated by wild-type NS5A-His was arbitrary fixed at 100. The results (triplicates) are representative of two independent experiments.