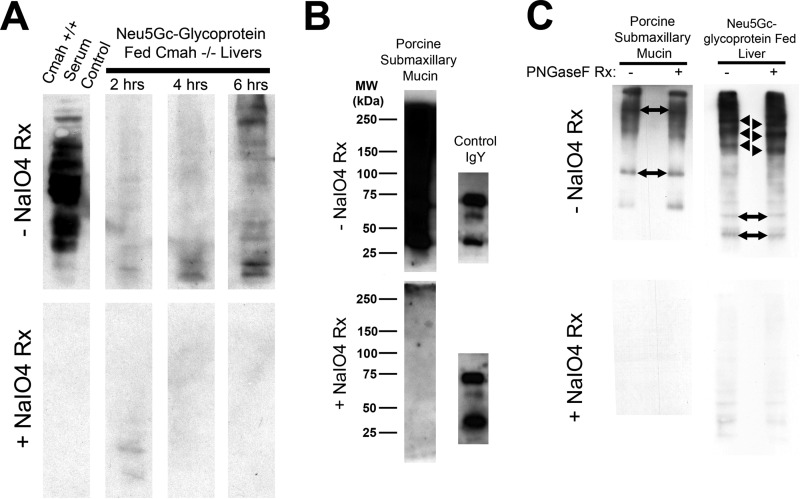
FIGURE 3.
Immunoblots with αNeu5Gc IgY confirm that Neu5Gc from Neu5Gc-glycoprotein feeding is incorporated into endogenous liver glycans. A, immunoblot of Neu5Gc-glycoprotein-fed Cmah−/− liver homogenates (25 μg/lane) at the indicated time point with αNeu5Gc IgY with (bottom) and without (top) periodate treatment (±NaIO4 reaction) of PVDF blotting membrane. Neu5Gc is present on many endogenous liver glycoproteins and signal increases over time. To control for staining, we treated control PVDF membranes with mild periodate (2 mm), which makes Neu5Gc unrecognizable to αNeu5Gc IgY. The positive control, Cmah+/+ Serum Control (left lane), is strong, but 100% sensitive to mild periodate treatment (bottom blot). B, immunoblot of native porcine submaxillary mucin (“Neu5Gc-glycoprotein,” 100 ng/lane) with αNeu5Gc IgY, with (bottom) and without (top) mild periodate treatment (as in A). PSM runs as a high molecular weight smear, with no apparent distinct bands. Chicken IgY (100 ng) was included to show that mild periodate treatment does not interfere with epitope recognition of proteins in SDS-PAGE. C, immunoblot of Neu5Gc-glycoprotein-fed Cmah−/− liver at 6 h with αNeu5Gc IgY with (bottom) and without (top) periodate treatment. To show that Neu5Gc is bioavailable for endogenous glycan synthesis, we used PNGase-F treatment of liver homogenates. PNGase-F treatment did not remove bands but instead leads to a shift toward a lower apparent molecular weight for several bands (black arrowhead) in +PNGase-F lanes, compared with −PNGase-F lanes. Migration of other bands within the same homogenate was not affected by PNGase-F treatment (two-sided black arrows). PSM controls exhibited no apparent shift with PNGase-F treatment, commensurate with mucin-type O-GalNAc glycans on PSM (B). Rx means reaction.