Background: Bispecific antibodies in cancer therapy operate through simultaneous binding of tumor cells and T cells.
Results: Bispecific antibodies with chemically programmed tumor cell specificity through a C-terminal selenocysteine residue kill tumor cells potently and specifically by recruiting and activating T cells.
Conclusion: Chemically programmed bispecific antibodies are active.
Significance: Chemically programmed bispecific antibodies have a broad utility in cancer therapy.
Keywords: Antibody Engineering, Biotechnology, Cancer Therapy, Immunology, Immunotherapy, Bispecific Antibodies
Abstract
Bispecific antibodies (biAbs) that mediate cytotoxicity by recruiting and activating endogenous immune cells are an emerging class of next-generation antibody therapeutics. Of particular interest are biAbs of relatively small size (∼50 kDa) that can redirect cytotoxic T cells through simultaneous binding of tumor cells. Here we describe a conceptually unique class of biAbs in which the tumor cell specificity of a humanized antibody fragment that recognizes CD3 on T cells is chemically programmed through a C-terminal selenocysteine (Sec) residue. We demonstrate that through chemically programmed specificity for integrin α4β1 or folate receptor 1 (FOLR1), and common specificity for CD3, these hybrid molecules exert potent and specific in vitro and ex vivo cytotoxicity toward tumor cell lines and primary tumor cells in the presence of primary T cells. Importantly, the generic nature of chemical programming allows one to apply our approach to virtually any specificity, promising a broad utility of chemically programmed biAbs in cancer therapy.
Introduction
As mAbs have captured a considerable market share in cancer therapy over the past 15 years, substantial efforts in industry, academia, and government are aimed toward conceptualizing and investigating next-generation antibody therapeutics with increased specificity and potency. The objective is to improve the clinical performance of mAbs with respect to durable complete responses and cures and to enable mAb therapy in more cancers and cancer patients.
Promising next-generation antibody therapeutics (1, 2) include biAbs2 that exert cytotoxicity by recruiting and activating endogenous immune cells. This can be achieved by combining antigen binding specificities for target cells (i.e. tumor cells) and effector cells (i.e. T cells, NK cells, and macrophages) in one molecule (3–5). For example, biAbs that recruit and activate T cells through CD3 of the T-cell receptor complex to instruct lysis of CD19-expressing malignant B cells has been an ongoing campaign for two decades (6), but these efforts have been hampered by production challenges regarding quantity, quality, and stability. Newer biAb formats, such as BiTE (bi-specific T cell engager) (7) and DART (dual affinity retargeting) (8) overcame these hurdles by reducing both the size and complexity of antibody molecule pairs. Several in vitro and in vivo studies have demonstrated that subnanomolar concentrations of BiTEs and DARTs selectively activate T cells to kill tumor cells (9). Furthermore, current phase I and II clinical trials with the (CD19 × CD3) BiTE blinatumomab have revealed impressive clinical activity at doses several orders of magnitude below those administered in conventional mAb therapy (10, 11). In addition to bypassing MHC/peptide recognition, T cells recruited by BiTEs and DARTs do not require ex vivo prestimulation or in vivo costimulation but, rather, are dependent on the presence of biAb-decorated tumor cells for activation. These favorable features of the BiTE and DART formats are attributed to their small size (∼50 kDa), which brings target and effector cells into close proximity to enable cytolytic synapses, and the monovalent engagement of the TCR complex, which prevents systemic activation of effector cells in the absence of target cells (9).
Although employing a variety of formats, recognition of cell surface receptors in conventional mAbs and biAbs is always mediated by Ig scaffolds. In addition to alternative protein scaffolds (12), an increasing number of peptides, peptidomimetics, and other small molecules rival Ig scaffolds with respect to both specificity and affinity. This is due, in part, to improved methods for rational design and the ability to generate and screen large small molecule libraries (13, 14). To equip small molecules with the pharmacokinetic and pharmacodynamic properties of mAbs, in particular, extended circulatory half-life and effector functions, chemical programming strategies have been developed that allow molecularly defined covalent docking of monospecific (15, 16) or bispecific (17, 18) small molecules that recognize cell surface receptors to antibody molecules with unique chemical reactivity. In addition to blending favorable features of small molecules and mAbs, chemically programmed mAbs are economically attractive because they utilize the same antibody construct for a virtually unlimited number of specificities, reducing production costs and shortening preclinical-to-clinical translation times (19).
Here we introduce the concept of chemically programmed biAbs that can recruit and activate T cells and are compatible with targeting any cell surface receptor that is recognizable by a small molecule. Exploiting the advantages of small size and monovalence, the invariable biological component of our concept is a ∼50-kDa humanized Fab molecule that binds to CD3 and contains a C-terminal Sec residue through which a variable chemical component can be covalently conjugated. Thus, the resulting conjugates are bispecific as they combine two different specificities in one molecule; one for CD3 mediated by the biological component and one for a cell surface receptor mediated by the chemical component. This work builds on our previously introduced chemical programming strategy that employs a Sec interface for the generation of molecularly defined antibody-small molecule conjugates in various formats (16, 20). To demonstrate the feasibility and potency of chemically programmed biAbs, we first synthesized derivatives of small molecules (supplemental Fig. S1) known to bind with high specificity and affinity to integrin α4β1 and FOLR1, which are expressed on the tumor cell surface in hematologic and solid malignancies, respectively.
Integrin α4β1 (also known as VLA-4) is a noncovalent heterodimer of two type I membrane proteins, ITGA4 (CD49D) and ITGB1 (CD29), that is selectively expressed in hematopoietic cells (21). It controls lymphocyte trafficking and homing by binding to vascular cell adhesion molecule 1 (VCAM1) and fibronectin (FN1). Furthermore, integrin α4β1 is expressed on the surface of malignant B cells, such as in mantle cell lymphoma (MCL), where it contributes to cell adhesion-mediated chemotherapy resistance (22). LLP2A is a peptidomimetic that was selected previously from a one-bead-one-compound chemical library for binding to integrin α4β1 (23). The one-bead-one-compound chemical library from which LLP2A originated was on the basis of mimicking the LDV tripeptide motif of FN1, which is recognized by integrin α4β1 (23). Notably, the LDV (i.e. LLP2A) binding site on integrin α4β1 is only accessible when the heterodimer is in its open, functionally active conformation that is provoked by inside-out signaling (21). Thus, LLP2A is a probe that detects the open conformation of integrin α4β1 (23, 24). When analyzing normal human peripheral blood mononuclear cells (PBMC) with LLP2A-biotin (25) and phycoerythrin (PE)-conjugated streptavidin in pilot flow cytometry studies, we did not detect binding to circulating B, T, and NK cells. By contrast, we observed strong binding to primary MCL cells and MCL cell lines. Thus, targeting the open conformation of integrin α4β1 with chemically programmed biAbs provides a compelling strategy for selective intervention of MCL and other hematologic malignancies.
FOLR1 (also known as folate receptor α) is an investigational target of mAbs (26) and small molecules (27) in clinical trials for the therapy of lung cancer, ovarian cancer, and other solid malignancies. FOLR1 is a high-affinity cellular entry receptor for folate (also known as vitamin B9), which is essential for DNA synthesis, repair, and methylation, i.e. cell growth, division, and differentiation. Tumor cells express FOLR1 to effectively compete for folate. Folate starvation can induce the cell surface expression of FOLR1 in epithelial tumor cell lines, such as the cervical carcinoma cell line HeLa (28). Thus, through different culture conditions, FOLR1-positive (FOLR1+) and FOLR1-negative (FOLR1-) HeLa cells can be generated, providing an ideal experimental system for investigating FOLR1 targeting in vitro.
EXPERIMENTAL PROCEDURES
Chemical Compounds
Primary Cells and Cell Lines
Human PBMC from healthy volunteers who gave written informed consent were prepared from freshly drawn blood obtained from the Department of Transfusion Medicine, Clinical Center, National Institutes of Health. PBMC were prepared by density gradient centrifugation using lymphocyte separation medium (MP Biomedicals) and used freshly. Human primary MCL cells were derived from a lymph node biopsy of an MCL patient enrolled in a National Institutes of Health clinical protocol with written informed consent and approval from the National Institutes of Health Institutional Review Board. The lymph node was disaggregated to single cells by passage through a wire mesh and resuspension in 10% (v/v) FCS in RPMI 1640 medium followed by cryopreservation in recovery cell culture freezing medium (Invitrogen). Human MCL cell line JeKo-1, human T-cell line Jurkat, and human cervical carcinoma cell line HeLa (ATCC) were maintained in 10% (v/v) FCS in regular RPMI 1640 (JeKo-1, Jurkat, FOLR1- HeLa) or folic acid-deficient RPMI 1640 medium (FOLR1+ HeLa) supplemented with penicillin-streptomycin (all from Invitrogen).
Cloning, Expression, and Purification of v9 IgG-Sec and Fab-Sec
On the basis of published amino acid sequences (29, 30), optimized DNA sequences encoding the variable domain of the heavy chain (VH) and kappa light chain (Vκ) of the humanized anti-human CD3 mAb v9 were custom synthesized (DNA2.0) and cloned by SacI/ApaI and HindIII/XbaI ligation, respectively, into the previously described mammalian cell expression vector PIGG-rituximab-Sec-His (20), affording PIGG-v9-IgG-Sec-His. To shorten the IgG expression cassette to a Fab expression cassette, an ApaI/SalI fragment of the previously described mammalian cell expression vector PIGG-rituxifab-Sec-His (20) was cloned into PIGG-v9-IgG-Sec-His by ApaI/SalI ligation, affording PIGG-v9-Fab-Sec-His. The mammalian cell expression vectors encoding v9 IgG-Sec and v9 Fab-Sec were transiently transfected into HEK 293F cells, and supernatants were collected, filtered, and concentrated as described (20). Subsequently, v9 IgG-Sec was purified by protein G affinity chromatography followed by immobilized metal affinity chromatography, and v9 Fab-Sec was purified by immobilized metal affinity chromatography as described (20). The purity of v9 IgG-Sec and v9 Fab-Sec was confirmed by SDS-PAGE followed by Coomassie staining, and the concentration was determined by absorbance at 280 nm.
Selective Conjugations
Established conditions for selective conjugation at the Sec interface were used (16, 20). In brief, v9 Fab-Sec, v9 IgG-Sec and the previously described (16) Fc-Sec were diluted in 15 ml of 100 mm sodium acetate (pH 5.2) and concentrated to 4 μm using a 10-kDa cutoff centrifugal filter device (Millipore). DTT at 0.1 mm followed by either LLP2A-biotin-maleimide, (LLP2A)2-biotin-maleimide, folate-biotin-maleimide (supplemental Fig. S1), or biotin-maleimide (maleimide-PEO2-biotin, Pierce) at 40 μm was added to the protein and incubated for 1 h at room temperature in the dark. Following conjugation, the proteins were diluted in 15 ml of 100 mm sodium acetate (pH 5.2) and concentrated to 250 μl as described above. This step was repeated once with 15 ml of 100 mm sodium acetate (pH 5.2) and subsequently twice with 15 ml of PBS to remove unconjugated compounds. Following sterile filtration, purified conjugates in PBS were stored refrigerated (4 °C) for short-term use and frozen (−80 °C) in aliquots for long-term use.
Integrin α4β1 Specificity ELISA
For coating, each well of a 96-well Costar 3690 plate (Corning) was incubated with 100 ng recombinant human integrin α4β1, α4β7, or α9β1 (R&D Systems) in 25 μl of TBS supplemented with 1 mm MnCl2 (Sigma-Aldrich) for 1 h at 37 °C. After blocking with 150 μl 3% (w/v) BSA/TBS (1 mm MnCl2) for 1 h at 37 °C, 50 ng/well v9 Fab-Sec/LLP2A-biotin, v9 Fab-Sec/(LLP2A)2-biotin, or v9 Fab-Sec in 1% (w/v) BSA/TBS (1 mm MnCl2) were added and incubated for 2 h at 37 °C. The plate was then washed with H2O and incubated with 50 μl of a 1:1000 dilution of HRP-conjugated goat anti-human kappa light chain pAbs (Southern Biotech) in 1% (w/v) BSA/TBS (1 mm MnCl2) for 1 h at 37 °C. Washing with H2O was repeated and colorimetric detection was performed using 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (Roche) as HRP substrate according to the manufacturer's directions. The absorbance at 405 nm was measured in a SpectraMax M5 microplate reader with SoftMax Pro software (Molecular Devices).
Interferon-γ Sandwich ELISA
For coating, each well of a 96-well Costar 3690 plate was incubated with 100 ng of mouse anti-human interferon-γ mAb 2G1 (Pierce) in 50 μl of PBS for 1 h at 37 °C. After blocking with 150 μl of 4% (w/v) BSA/PBS for 1 h at 37 °C, recombinant human interferon-γ (Pierce) at 0–2500 pg/ml in 4% (w/v) BSA/PBS (50 μl/well) or the supernatants (50 μl/well) from the CD69 assay described below were added and incubated for 2 h at 37 °C. Without washing, 10 ng/50 μl biotin-conjugated mouse anti-human interferon-γ mAb B133.5 (Pierce) in 4% (w/v) BSA/PBS was added to each well and incubated for 1 h at 37 °C. After washing with 0.2% (v/v) Tween 20 in PBS, 50 μl of a 1:1000 dilution of HRP-conjugated streptavidin (Southern Biotech) in 4% (w/v) BSA/PBS was added to each well and incubated for 1 h at 37 °C. After washing with 0.2% (v/v) Tween 20 in PBS, colorimetric detection and measurement was carried out as described above. The correlation of absorbance (y) and concentration (x) closely fit (r2 = 0.9973) a linear regression line (y = 0.0038x + 0.0179), which was used to calculate the interferon-γ concentration in the supernatants.
Flow Cytometry, PBMC
Freshly prepared human PBMC from healthy volunteers were first incubated for 15 min on ice in undiluted human AB serum (Invitrogen) to block Fcγ receptors. Blocked human PBMC were diluted to 5 × 105 cells in 10% (v/v) human AB serum in PBS and then incubated with 2 μg/ml v9 Fab-Sec/LLP2A-biotin, 2 μg/ml Fc-Sec/LLP2A-biotin, or an equimolar concentration (40 nm) of LLP2A-biotin (25) for 1 h on ice in a total volume of 50 μl. After washing twice with 2% (v/v) human AB serum in PBS, the cells were incubated with 2 μg/ml PE-conjugated streptavidin (BD Biosciences) and APC-conjugated costaining mAbs (see below) for 30 min on ice, washed twice as before, and resuspended in 400 μl of 2% (v/v) human AB serum in PBS. PBMC subpopulations were gated by costaining with APC-conjugated mouse anti-human CD4, CD8, CD16, CD19, and CD56 mAbs (all from BD Biosciences) according to the directions of the manufacturer. Propidium iodide (Invitrogen) was added to a final concentration of 5 μg/ml to exclude dead cells from the analysis. Cells were analyzed using a FACSCalibur instrument (BD Biosciences) and FlowJo analytical software (Tree Star). Following trypsinization with 0.25% (w/v) trypsin (Invitrogen), FOLR1+ and FOLR1- HeLa cells were collected by centrifugation, resuspended in 1% (v/v) FCS/PBS, and aliquots of 50 μl containing 5 × 105 cells were distributed into a V-bottom 96-well plate (Corning). The cells were then incubated with 5 μg/ml v9 Fab-Sec/folate-biotin or Fc-Sec/folate-biotin for 45 min on ice. After washing twice with 1% (v/v) FCS/PBS, the cells were incubated with 2 μg/ml of PE-conjugated streptavidin for 45 min on ice. After washing twice as before, the cells were resuspended in 400 μl 1% (v/v) FCS/PBS and analyzed by flow cytometry as described above.
CD69 Assay
FOLR1+ and FOLR1- HeLa cells were used as target cells and distributed into 96-well U-bottom plates (Corning) at a density of 5 × 104 cells/50 μl/well. Freshly prepared human PBMC were used as effector cells and added (50 μl/well) to the target cells at an effector-to-target cell ratio of 20:1. After incubation for 16 h at 37 °C in 5% CO2 with or without v9 Fab-Sec/folate-biotin at 2 μg/ml, the supernatant was used to detect the expression of interferon-γ by a sandwich ELISA as described above. The cells were incubated with a PE-conjugated mouse-anti-human CD69 mAb (BD Biosciences) and a mixture of APC-conjugated mouse anti-human CD4 and CD8 mAbs (BD Biosciences) according to the directions of the manufacturer for 30 min on ice. After washing twice with 1% (v/v) FCS/PBS, the cells were resuspended in 400 μl of 1% (v/v) FCS/PBS and analyzed by flow cytometry as described above.
Cell-Cell Association Assay
JeKo-1 cells were labeled with CSFE using the CellTrace CFSE cell proliferation kit (Invitrogen) according to the directions of the manufacturer. Simultaneously, Jurkat cells were labeled with PKH26 using the PKH26 red fluorescent cell linker kit (Sigma-Aldrich) according to the directions of the manufacturer. Cells (5 × 106/ml) from each cell line were resuspended in PBS and mixed in a 1:1 ratio in the presence of 2 μg/ml v9 Fab-Sec/LLP2A-biotin or v9 Fab-Sec/(LLP2A)2-biotin and incubated for 30 min at room temperature. In parallel, 2 μg/ml v9 Fab-Sec/biotin, Fc-Sec/LLP2A-biotin, and Fc-Sec/(LLP2A)2-biotin were used as negative controls. After resuspending the cells in 1% paraformaldehyde in PBS, cell-cell associations indicated by a CFSE+ PKH26+ double-positive population were detected and quantified by flow cytometry as described above.
Cell Adhesion Assays
All incubations were for 1 h at 37 °C. A 96-well Costar 3690 plate was coated with 1 μg of recombinant human integrin α4β1 or recombinant human FOLR1 (R&D Systems) in 25 μl of TBS and blocked with 3% (w/v) BSA/TBS. For integrin α4β1, 1 mm MnCl2 was added to TBS. Jurkat cells (1 × 105 cells in 50 μl of PBS) were incubated with 10 μg/ml v9 Fab-Sec/LLP2A-biotin, v9 Fab-Sec/(LLP2A)2-biotin, or v9 Fab-Sec/folate-biotin and added to the prepared plate. In parallel, 10 μg/ml v9 Fab-Sec/biotin, Fc-Sec/LLP2A-biotin, Fc-Sec/(LLP2A)2-biotin, and Fc-Sec/folate-biotin were used as negative controls and the absence of conjugates as background. Non-adherent cells were removed by gently washing twice with PBS. The relative number of adherent cells was determined through a colorimetric lactate dehydrogenase assay using the CytoTox 96 non-radioactive cytotoxicity assay kit (Promega) according to the directions of the manufacturer. The absorbance at 490 nm was measured in a SpectraMax M5 microplate reader with SoftMax Pro software.
Cytotoxicity Assays
Cytotoxicity was measured with a bioluminescent protease release assay (CytoTox-Glo cytotoxicity assay, Promega) using the protocol of the manufacturer with minor modifications. Freshly prepared human PBMC from healthy volunteers were used as effector cells. JeKo-1 cells, human primary MCL cells, FOLR1+ HeLa cells, and FOLR1- HeLa cells were used as target cells and distributed into 96-well U-bottom plates at a density of 5 × 104 cells/well (50 μl/well). Subsequently, v9 Fab-Sec/LLP2A-biotin, v9 Fab-Sec/(LLP2A)2-biotin, v9 Fab-Sec/folate-biotin, or v9 IgG-Sec/folate-biotin were added to the target cells over a concentration range of 0.02–20 μg/ml. In parallel, v9 Fab-Sec/biotin, Fc-Sec/LLP2A-biotin, Fc-Sec/(LLP2A)2-biotin, v9 IgG-Sec/biotin, and Fc-Sec/folate-biotin were used as negative controls. Without washing, effector cells were added (50 μl/well) at an effector-to-target (E:T) cell ratio of 20:1, or from 1:1 to 30:1, and incubated for 16 h at 37 °C in 5% CO2. After centrifugation, 50 μl/well of supernatant was transferred to a 96-well white clear-bottom Costar 3610 plate (Corning) followed by addition of 25 μl/well CytoTox-Glo cytotoxicity assay reagent. After 15 min at room temperature, luminescence was measured in a SpectraMax M5 microplate reader with SoftMax Pro software. The percentage of specific cytotoxicity was calculated according to the following formula: Percent specific cytotoxicity = 100 × (EX - Espon - Tspon)/(Tmax - Tspon), where EX represents the release from experimental wells, Espon is the spontaneous release from effector cells alone, Tspon is the spontaneous release from target cells alone, and Tmax is the maximum release from target cells lysed in 30 μg/ml digitonin. Data were computed as mean ± S.D. of triplicates. An unpaired two-tailed Student's t test was used to calculate p.
RESULTS
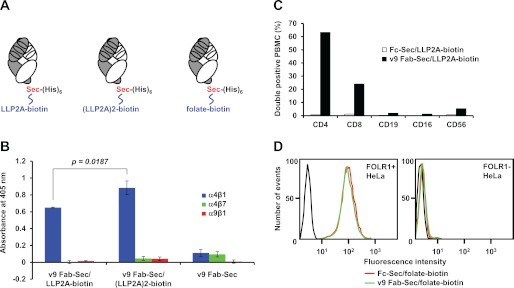
We cloned, expressed, and purified the previously described (30) humanized mouse anti-human CD3 mAb, v9, in the 50-kDa Fab format with a Sec at the C-terminus of the heavy chain fragment followed by a hexa-histidine tag. The yield of purified v9 Fab-Sec from supernatants of transiently transfected HEK 293F cells was 1–2 mg/liter. Using mildly acidic and reducing conditions we had established previously for selective Sec conjugation without modifying cysteines or other amino acids in the antibody molecule (16), v9 Fab-Sec was conjugated to monovalent and bivalent LLP2A-biotin derivatives via their maleimide functionality (supplemental Fig. S1). The resulting conjugates, v9 Fab-Sec/LLP2A-biotin and v9 Fab-Sec/(LLP2A)2-biotin (Fig. 1A), were tested by ELISA for selective binding to the open conformation of integrin α4β1 (B). In the presence of 1 mm MnCl2, which stabilizes the open conformation, both conjugates selectively bound to immobilized human integrin α4β1 but not to α4β7 and α9β1. A higher avidity for the bivalent compound was evident in the significantly higher ELISA signal obtained for v9 Fab-Sec/(LLP2A)2-biotin versus v9 Fab-Sec/LLP2A-biotin. Next, we tested both conjugates for selective binding to cell surface CD3 on T cells. Human PBMC from a normal donor were stained with v9 Fab-Sec/LLP2A-biotin or Fc-Sec/LLP2A-biotin followed by PE-conjugated streptavidin and APC-conjugated mouse anti-human CD4, CD8, CD19, CD16, or CD56 mAbs for T cell, B cell, and NK cell gating. Notably, CD4+ and CD8+ T cells, but not CD19+ B cells and CD16+ NK cells, were recognized by v9 Fab-Sec/LLP2A-biotin (Fig. 1C). The small numbers of double-stained CD56+ cells likely are NKT cells which also express CD3. Finally, control conjugate Fc-Sec/LLP2A-biotin did not bind to any gated cells, confirming the absence of the open conformation of integrin α4β1 on circulating lymphocytes. Through conjugation to a folate-biotin derivative with maleimide functionality (supplemental Fig. S1), both Fc-Sec and v9 Fab-Sec acquired FOLR1 specificity, as demonstrated by flow cytometry with FOLR1+ and FOLR1- HeLa cells (Fig. 1D). Thus, v9 Fab-Sec can be chemically programmed for recognizing diverse cell surface receptors.
FIGURE 1.
Concept and specificity of chemically programmed biAbs. A, drawing of anti-human CD3 Fab v9 with a Sec at the C-terminus of the heavy chain fragment followed by a hexa-histidine tag and chemically programmed with LLP2A-biotin (left panel), (LLP2A)2-biotin (center panel) for human integrin α4β1 binding, or folate-biotin (right panel) for human FOLR1 binding. The light chain is shown in white and the heavy chain fragment in gray. Each consists of one variable and one constant Ig domain. Also indicated are the three complementarity determining regions of each variable domain that mediate CD3 binding. B, binding of v9 Fab-Sec before (right) or after chemical programming with LLP2A-biotin (left) or (LLP2A)2-biotin (center) to immobilized human integrins α4β1 (blue), α4β7 (green), and α9β1 (red) in the presence of 1 mm MnCl2 as detected with HRP-conjugated goat anti-human κ light chain polyclonal antibodies (pAbs). Shown are mean values of triplicates ± S.D.. An unpaired two-tailed Student's t test was used to calculate p. C, binding of v9 Fab-Sec/LLP2A-biotin (black) detected with PE-conjugated streptavidin to normal human PBMC subpopulations gated with APC-conjugated mouse anti-human CD4, CD8, CD19, CD16, and CD56 mAbs. Shown is the percentage of cells in the double positive quadrant. Fc-Sec/LLP2A-biotin (white) was used as negative control. D, flow cytometry analysis of the binding of v9 Fab-Sec/folate-biotin (green), Fc-Sec/folate-biotin (red), or the detecting reagent PE-conjugated streptavidin alone (black) to FOLR1+ (left panel) and FOLR1- (right panel) HeLa cells.
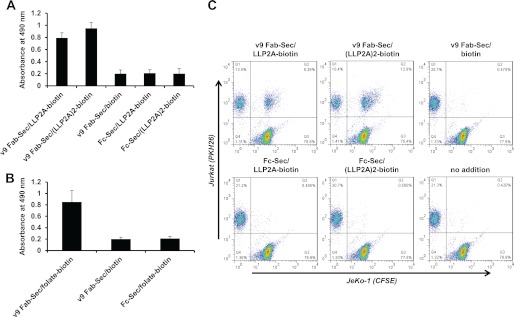
Next, we demonstrated the simultaneous recognition of target and effector cell surface receptors by chemically programmed v9 Fab-Sec. Through testing the ability of v9 Fab-Sec chemically programmed with LLP2A-biotin, (LLP2A)2-biotin, and folate-biotin to cross-link CD3+ Jurkat cells to immobilized integrin α4β1 (Fig. 2A) and FOLR1 (B), respectively, we revealed bispecificity in the three chemically programmed biAbs, but not in various control conjugates. Furthermore, we showed cell-cell associations between CD3+ Jurkat cells and JeKo-1 cells, an MCL cell line that expresses the open conformation of integrin α4β1, in the presence of v9 Fab-Sec/LLP2A-biotin and v9 Fab-Sec/(LLP2A)2-biotin but not in the presence of control conjugates (Fig. 2C). As expected, the bivalent compound was more potent than the monovalent compound in mediating cross-linking and cell-cell association.
FIGURE 2.
Bispecificity of chemically programmed biAbs. A, adherence of CD3+ Jurkat cells incubated with 10 μg/ml bispecific conjugates (v9 Fab-Sec/LLP2A-biotin and v9 Fab-Sec/(LLP2A)2-biotin) and control conjugates (v9 Fab-Sec/biotin, Fc-Sec/LLP2A-biotin, and Fc-Sec/(LLP2A)2-biotin) to immobilized human integrin α4β1 in the presence of 1 mm MnCl2. B, adherence of CD3+ Jurkat cells incubated with 10 μg/ml bispecific conjugate (v9 Fab-Sec/folate-biotin) and control conjugates (v9 Fab-Sec/biotin and Fc-Sec/folate) to immobilized human FOLR1. In both experiments, the relative number of adherent cells was determined through a colorimetric lactate dehydrogenase assay. Shown are mean values of triplicates ± S.D. after subtraction of the background measured in the absence of conjugates. C, cell-cell association of CD3+ Jurkat cells loaded with red fluorescent dye PKH26 and integrin α4β1+ JeKo-1 cells loaded with green fluorescent dye CFSE. Cells were mixed in a 1:1 ratio and incubated for 30 min at room temperature in the absence or presence of 2 μg/ml bispecific conjugates (v9 Fab-Sec/LLP2A-biotin and v9 Fab-Sec/(LLP2A)2-biotin) and control conjugates (v9 Fab-Sec/biotin, Fc-Sec/LLP2A-biotin, and Fc-Sec/(LLP2A)2-biotin). Following resuspension in 1% (w/v) paraformaldehyde in PBS, double fluorescent Jurkat-JeKo-1 cell-cell associations were detected by flow cytometry (upper right quadrant).
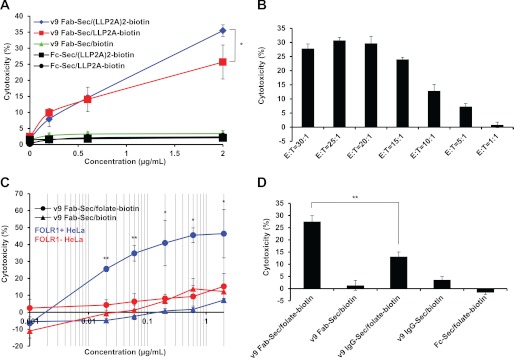
Having confirmed the bispecificity of chemically programmed v9 Fab-Sec, we next tested its ability to redirect cytotoxic T cells in vitro. Using normal human PBMC as effector cells (E) and JeKo-1 cells as target cells (T), we assessed the cytotoxicity of v9 Fab-Sec/LLP2A-biotin and v9 Fab-Sec/(LLP2A)2-biotin after 16-h incubation with concentrations ranging from 0.2–2 μg/ml (4–40 nm) at an E:T ratio of 20:1. Both conjugates revealed dose-dependent cytotoxicity, with the bivalent compound significantly outperforming the monovalent compound at the highest concentration (Fig. 3A). No cytotoxicity was detected for control conjugate v9 Fab-Sec/biotin. Two additional control conjugates, Fc-Sec/LLP2A-biotin and Fc-Sec/(LLP2A)2-biotin, also did not trigger cytotoxicity despite their theoretical potential of mediating antibody-dependent cellular cytotoxicity via the Fc domain. These findings were reproducible with human PBMC from six different normal donors. To demonstrate effector cell-dependent cytotoxicity, we performed the same experiment with 2 μg/ml v9 Fab-Sec/(LLP2A)2-biotin at E:T ratios ranging from 1:1 to 30:1 (Fig. 3B).
FIGURE 3.
Activity of chemically programmed biAbs. A, cytotoxicity of different concentrations of bispecific conjugates v9 Fab-Sec/(LLP2A)2-biotin (blue ♦) and v9 Fab-Sec/LLP2A-biotin (red ■) and control conjugates v9 Fab-Sec/biotin (green ▴), Fc-Sec/(LLP2A)2-biotin (black ■), and Fc-Sec/LLP2A-biotin (black ●), with normal human PBMC as effector cells (E) and JeKo-1 cells as target cells (T) at an E:T ratio of 20:1. B, cytotoxicity of 2 μg/ml v9 Fab-Sec/(LLP2A)2-biotin at the indicated seven E:T ratios. C, cytotoxicity of different concentrations of bispecific conjugate (v9 Fab-Sec/folate-biotin (●)) and control conjugate (v9 Fab-Sec/biotin (▴)) with normal human PBMC (E) and FOLR1+ (blue) and FOLR1- (red) HeLa cells (T) at an E:T ratio of 20:1. To support the log conversion, 0 μg/ml concentrations are shown as 0.001 μg/ml. D, comparison of the cytotoxicity of equimolar concentrations (4 nm) of v9 Fab-Sec/folate-biotin (0.2 μg/ml) and v9 IgG-Sec/folate-biotin (0.6 μg/ml) and control conjugates (v9 Fab-Sec/biotin (0.2 μg/ml), v9 IgG-Sec/biotin (0.6 μg/ml), and Fc-Sec/folate-biotin (0.2 μg/ml)) with normal human PBMC (E) and FOLR1+ HeLa cells (T) at an E:T ratio of 20:1. In all experiments, cytotoxicity was measured with the CytoTox-Glo cytotoxicity assay after a 16-h incubation at 37 °C. Shown are mean values of triplicates ± S.D.. An unpaired two-tailed Student's t test was used to calculate p. *, p < 0.05; **, p < 0.01, respectively. Actual p values were 0.0362 (A); from left to right, 0.0009, 0.0008, 0.0237, 0.0011, 0.0219 (C); and 0.0008 (D).
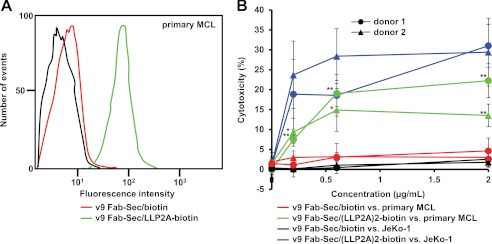
To investigate primary tumor cells from a lymph node biopsy of a MCL patient as target cells for chemically programmed biAbs, we first used flow cytometry to show recognition by v9 Fab-Sec/LLP2A-biotin but not v9 Fab-Sec/biotin, revealing expression of the open conformation of integrin α4β1 (Fig. 4A). Using normal human PBMC from two different donors and the same conditions as before, we then compared the cytotoxicity mediated by v9 Fab-Sec/(LLP2A)2-biotin and the negative control, v9 Fab-Sec/biotin, toward primary MCL cells (Fig. 4B). The chemically programmed biAb was found to mediate significant cytotoxicity at concentrations ranging from 0.2–2 μg/ml.
FIGURE 4.
Primary cell binding and cytotoxicity of chemically programmed biAbs. A, flow cytometry analysis of the binding of v9 Fab-Sec/LLP2A-biotin (green), v9 Fab-Sec/biotin (red), or the detecting reagent PE-conjugated streptavidin alone (black) to primary MCL cells from a lymph node biopsy. B, cytotoxicity of different concentrations of bispecific conjugate v9 Fab-Sec/(LLP2A)2-biotin toward primary MCL cells (green) and JeKo-1 cells (blue) as target cells (T) in the presence of normal human PBMC from donor 1 (●) and donor 2 (▴) as effector cells (E). The cytotoxicity of corresponding concentrations of control conjugate v9 Fab-Sec/biotin toward primary MCL cells (red) and JeKo-1 cells (black) was determined in parallel. In all experiments, cytotoxicity was measured with the CytoTox-Glo cytotoxicity assay after a 16-h incubation at 37 °C and an E:T ratio of 20:1. Shown are mean values of triplicates ± S.D. For the cytotoxicity of v9 Fab-Sec/(LLP2A)2-biotin versus v9 Fab-Sec/biotin toward primary MCL cells (i.e. green versus red), an unpaired two-tailed Student's t test was used to calculate p. *, p < 0.05; **, p < 0.01, respectively. Actual p values were (● from left to right) 0.002, 0.0022, 0.0029 and (▴ from left to right) 0.0354, 0.0293, 0.0073.
The activities of v9 Fab-Sec/folate-biotin and the negative control, v9 Fab-Sec/biotin, were determined over a concentration range of 0.02–2 μg/ml (0.4–40 nm) using the same conditions as before with FOLR1+ and FOLR1- HeLa cells as target cells (Fig. 3C). Only the chemically programmed biAb was found to mediate significant cytotoxicity toward FOLR1+ HeLa cells, even at subnanomolar concentrations. FOLR1- HeLa cells were not killed. To compare the influence of antibody format (i.e. Fab versus IgG) on chemically programmed biAbs, the same experiment was carried out with equimolar concentrations (4 nm) of v9 Fab-Sec/folate-biotin and v9 IgG-Sec/folate-biotin in addition to the corresponding negative controls v9 Fab-Sec/biotin, v9 IgG-Sec/biotin, and Fc-Sec/folate-biotin (Fig. 3D). Notably, the chemically programmed biAb in the ∼50-kDa Fab format significantly outperformed the ∼150-kDa IgG format.
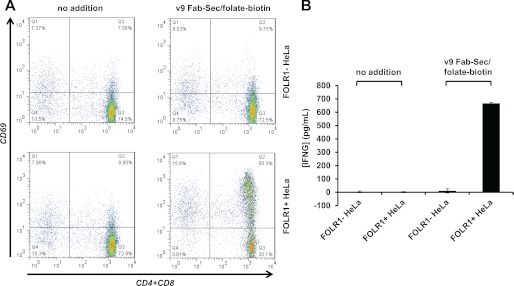
To examine the mechanism of cytotoxicity, we analyzed the cell surface expression of activation marker CD69 on the T cell fraction of PBMC (Fig. 5A). After coculture with FOLR1+ HeLa cells in the presence of 2 μg/ml v9 Fab-Sec/folate-biotin, a majority of T cells expressed CD69. By contrast, no up-regulation of CD69 was found in the absence of the chemically programmed biAb or after coculture with FOLR1- HeLa cells. Thus, T cells were only activated in the presence of chemically programmed biAb bound to tumor cells. Selective T cell activation in the presence of chemically programmed biAb bound to tumor cells was further shown by determining the concentration of interferon-γ in the supernatants of the same experiment (Fig. 5B).
FIGURE 5.
T cell activation by chemically programmed biAbs. A, cell surface expression of activation marker CD69 on the T cell fraction of PBMC (E) cocultured for 16 h with FOLR1- and FOLR1+ HeLa cells (T) at an E:T ratio of 20:1 in the presence or absence of 2 μg/ml v9 Fab-Sec/folate-biotin. T cells were stained with a mixture of APC-conjugated mouse anti-human CD4 and CD8 mAbs (x axis) and PE-conjugated mouse anti-human CD69 mAb (y axis). B, concentration of interferon-γ (INFG) in the supernatants of the same experiment detected and quantified with a sandwich ELISA. Shown are mean values of triplicates ± S.D.
DISCUSSION
Chemical programming requires the presence of a unique chemical reactivity center in the antibody molecule through which small molecules can be covalently docked to yield site-specific (as opposed to random) conjugates. We have demonstrated previously that a C-terminal Sec residue provides a suitable reactivity center for chemical programming (16, 20). In this study we show that a humanized anti-human CD3 Fab with a C-terminal Sec residue can serve as invariable biological component that recognizes T cells and can be chemically programmed for tumor cell specificity with a variable chemical component. Using three different chemical components we show that the ensuing chemically programmed biAbs recruit and activate T cells for potent and specific tumor cell killing.
In a previous study, random conjugation of a peptide to a mouse anti-human CD3 mAb in IgG format yielded a biAb that killed tumor cells in the presence of T cells (31). Furthermore, random conjugation of folate to mouse anti-human CD3 mAbs in IgG, Fab, and scFv formats generated active biAbs (32, 33). However, a shortcoming of random conjugation through the ϵ-amino group of lysine residues in these studies or the thiol group of cysteine residues in other studies is the fact that the resulting antibody conjugate is a mixture of molecules with a range of stoichiometries and characterized by substantial batch-to-batch variability. Furthermore, random conjugation can impair the antigen binding site, in particular in smaller sized antibody fragments, and is heavily influenced by properties of the small molecule, such as size, charge, and solubility. For these reasons, random conjugation is not suitable for chemical programming, which mandates a generic assembly process of a molecularly defined conjugate that does not interfere with the function of the invariable biological component and is largely independent of the structural diversity of the variable chemical component.
We chose the Fab format for chemically programmed biAbs because it resembles biAbs in the BiTE and DART format with respect to size and valence. These properties are thought to be critical for the formation of cytolytic synapses between tumor cell and T cell and for the prevention of systemic activation of T cells in the absence of tumor cells. In fact, in our direct comparison chemically programmed biAbs in Fab format significantly outperformed the corresponding IgG format. In addition, the Fab format did not activate T cells in the absence of tumor cells. Although monovalent T cell engagement is critical for this systemic silence, binding to tumor cells could benefit from the avidity of bivalent engagement. Using LLP2A and (LLP2A)2 for proof of concept, we demonstrate that a conversion from monovalent to bivalent engagement can be achieved through chemical synthesis without substantially increasing the size of chemically programmed biAbs. In addition to bivalent monospecific chemical components, such as (LLP2A)2, bivalent bispecific chemical components could be synthesized to simultaneously engage a second tumor cell surface receptor, effectively delivering a chemically programmed trispecific antibody. Likewise, the chemical compound could incorporate a moiety that extends the circulatory half-life of chemically programmed biAbs in Fab format, which is expected to be as short as that of BiTEs and DARTs. Suitable moieties for extending the circulatory half-life include polyethylene glycol polymers and peptides, peptidomimetics, or other small molecules that bind to serum proteins. Collectively, the ability to tailor valence, specificity, and, potentially, circulatory half-life through chemical synthesis is a distinctive asset of chemically programmed biAbs. Furthermore, compared with conventional mAbs, small molecules may be more adept at reaching concealed epitopes in membrane proximal locations for the formation of cytolytic synapses. Through the recognition of concealed epitopes, small molecules can also afford exceptional specificity as in the case of LLP2A and (LLP2A)2, which selectively recognize the open conformation of integrin α4β1.
Another distinctive asset of chemically programmed biAbs is their invariable biological component. The generic design of a humanized anti-human CD3 Fab with a C-terminal Sec enables the rapid deployment of small molecules with virtually any receptor specificity for the chemical programming of biAbs. Likewise, it is conceivable to deploy the invariable biological component for the screening of small molecule libraries toward optimizing the specificity, affinity, and activity of chemically programmed biAbs. The generic design may also facilitate combinations of two or more chemically programmed biAbs that bind to different receptors or different epitopes of the same receptor expressed on the tumor cell surface. Such cocktails could mediate enhanced cytotoxicity and prevent resistance mechanisms.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Hofer, Lauren R. Skeffington, Dr. Sivasubramanian Baskar, and Dr. Adrian Wiestner for their contributions.
This work was supported, in whole or in part, by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.

This article contains supplemental Fig. S1.
- biAbs
- bispecific antibodies
- Sec
- selenocysteine
- FOLR1
- folate receptor 1
- BiTE
- bispecific T cell engager
- DART
- dual affinity retargeting
- MCL
- mantle cell lymphoma
- PBMC
- peripheral blood mononuclear cell(s)
- PE
- phycoerythrin
- APC
- allophycocyanin.
REFERENCES
- 1. Carter P. (2001) Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 1, 118–129 [DOI] [PubMed] [Google Scholar]
- 2. Li J., Zhu Z. (2010) Research and development of next generation of antibody-based therapeutics. Acta Pharmacol. Sin. 31, 1198–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi B. D., Cai M., Bigner D. D., Mehta A. I., Kuan C. T., Sampson J. H. (2011) Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin. Biol. Ther. 11, 843–853 [DOI] [PubMed] [Google Scholar]
- 4. Holmes D. (2011) Buy buy bispecific antibodies. Nat. Rev. Drug Discov. 10, 798–800 [DOI] [PubMed] [Google Scholar]
- 5. Chames P., Baty D. (2009) Bispecific antibodies for cancer therapy. The light at the end of the tunnel? mAbs 1, 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson P. M., Crist W., Hasz D., Carroll A. J., Myers D. E., Uckun F. M. (1992) G19.4(alpha CD3) x B43(α CD19) monoclonal antibody heteroconjugate triggers CD19 antigen-specific lysis of t(4;11) acute lymphoblastic leukemia cells by activated CD3 antigen-positive cytotoxic T cells. Blood 80, 2826–2834 [PubMed] [Google Scholar]
- 7. Baeuerle P. A., Reinhardt C. (2009) Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 69, 4941–4944 [DOI] [PubMed] [Google Scholar]
- 8. Moore P. A., Zhang W., Rainey G. J., Burke S., Li H., Huang L., Gorlatov S., Veri M. C., Aggarwal S., Yang Y., Shah K., Jin L., Zhang S., He L., Zhang T., Ciccarone V., Koenig S., Bonvini E., Johnson S. (2011) Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 117, 4542–4551 [DOI] [PubMed] [Google Scholar]
- 9. Rader C. (2011) DARTs take aim at BiTEs. Blood 117, 4403–4404 [DOI] [PubMed] [Google Scholar]
- 10. Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S., Noppeney R., Viardot A., Hess G., Schuler M., Einsele H., Brandl C., Wolf A., Kirchinger P., Klappers P., Schmidt M., Riethmüller G., Reinhardt C., Baeuerle P. A., Kufer P. (2008) Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 [DOI] [PubMed] [Google Scholar]
- 11. Topp M. S., Kufer P., Gökbuget N., Goebeler M., Klinger M., Neumann S., Horst H. A., Raff T., Viardot A., Schmid M., Stelljes M., Schaich M., Degenhard E., Köhne-Volland R., Brüggemann M., Ottmann O., Pfeifer H., Burmeister T., Nagorsen D., Schmidt M., Lutterbuese R., Reinhardt C., Baeuerle P. A., Kneba M., Einsele H., Riethmüller G., Hoelzer D., Zugmaier G., Bargou R. C. (2011) Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 [DOI] [PubMed] [Google Scholar]
- 12. Löfblom J., Frejd F. Y., Ståhl S. (2011) Non-immunoglobulin-based protein scaffolds. Curr. Opin. Biotechnol. 22, 843–848 [DOI] [PubMed] [Google Scholar]
- 13. Kodadek T. (2010) Synthetic receptors with antibody-like binding affinities. Curr. Opin. Chem. Biol. 14, 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernardo P. H., Tong J. C. (2012) In silico design of small molecules. Methods Mol. Biol. 800, 25–31 [DOI] [PubMed] [Google Scholar]
- 15. Rader C., Sinha S. C., Popkov M., Lerner R. A., Barbas C. F., 3rd (2003) Chemically programmed monoclonal antibodies for cancer therapy. Adaptor immunotherapy based on a covalent antibody catalyst. Proc. Natl. Acad. Sci. U.S.A. 100, 5396–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hofer T., Thomas J. D., Burke T. R., Jr., Rader C. (2008) An engineered selenocysteine defines a unique class of antibody derivatives. Proc. Natl. Acad. Sci. U.S.A. 105, 12451–12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gavrilyuk J. I., Wuellner U., Salahuddin S., Goswami R. K., Sinha S. C., Barbas C. F., 3rd (2009) An efficient chemical approach to bispecific antibodies and antibodies of high valency. Bioorg. Med. Chem. Lett. 19, 3716–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doppalapudi V. R., Huang J., Liu D., Jin P., Liu B., Li L., Desharnais J., Hagen C., Levin N. J., Shields M. J., Parish M., Murphy R. E., Del Rosario J., Oates B. D., Lai J. Y., Matin M. J., Ainekulu Z., Bhat A., Bradshaw C. W., Woodnutt G., Lerner R. A., Lappe R. W. (2010) Chemical generation of bispecific antibodies. Proc. Natl. Acad. Sci. U.S.A. 107, 22611–22616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woodnutt G., Violand B., North M. (2008) Advances in protein therapeutics. Curr. Opin. Drug Discov. Devel. 11, 754–761 [PubMed] [Google Scholar]
- 20. Hofer T., Skeffington L. R., Chapman C. M., Rader C. (2009) Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry 48, 12047–12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai Y., Shimaoka M., Kurokawa M. (2010) Essential roles of VLA-4 in the hematopoietic system. Int. J. Hematol. 91, 569–575 [DOI] [PubMed] [Google Scholar]
- 22. Kurtova A. V., Tamayo A. T., Ford R. J., Burger J. A. (2009) Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d). Importance for interactions with the stromal microenvironment and specific targeting. Blood 113, 4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng L., Liu R., Marik J., Wang X., Takada Y., Lam K. S. (2006) Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat. Chem. Biol. 2, 381–389 [DOI] [PubMed] [Google Scholar]
- 24. Denardo S. J., Liu R., Albrecht H., Natarajan A., Sutcliffe J. L., Anderson C., Peng L., Ferdani R., Cherry S. R., Lam K. S. (2009) 111In-LLP2A-DOTA polyethylene glycol-targeting α4β1 integrin. Comparative pharmacokinetics for imaging and therapy of lymphoid malignancies. J. Nucl. Med. 50, 625–634 [DOI] [PubMed] [Google Scholar]
- 25. Thomas J. D., Hofer T., Rader C., Burke T. R., Jr. (2008) Application of a trifunctional reactive linker for the construction of antibody-drug hybrid conjugates. Bioorg. Med. Chem. Lett. 18, 5785–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spannuth W. A., Sood A. K., Coleman R. L. (2010) Farletuzumab in epithelial ovarian carcinoma. Expert Opin. Biol. Ther. 10, 431–437 [DOI] [PubMed] [Google Scholar]
- 27. Low P. S., Kularatne S. A. (2009) Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 13, 256–262 [DOI] [PubMed] [Google Scholar]
- 28. Antony A., Tang Y. S., Khan R. A., Biju M. P., Xiao X., Li Q. J., Sun X. L., Jayaram H. N., Stabler S. P. (2004) Translational up-regulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions. J. Clin. Invest. 113, 285–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shalaby M. R., Shepard H. M., Presta L., Rodrigues M. L., Beverley P. C., Feldmann M., Carter P. (1992) Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J. Exp. Med. 175, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu Z., Carter P. (1995) Identification of heavy chain residues in a humanized anti-CD3 antibody important for efficient antigen binding and T cell activation. J. Immunol. 155, 1903–1910 [PubMed] [Google Scholar]
- 31. Liu M. A., Nussbaum S. R., Eisen H. N. (1988) Hormone conjugated with antibody to CD3 mediates cytotoxic T cell lysis of human melanoma cells. Science 239, 395–398 [DOI] [PubMed] [Google Scholar]
- 32. Kranz D. M., Patrick T. A., Brigle K. E., Spinella M. J., Roy E. J. (1995) Conjugates of folate and anti-T-cell-receptor antibodies specifically target folate receptor-positive tumor cells for lysis. Proc. Natl. Acad. Sci. U.S.A. 92, 9057–9061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rund L. A., Cho B. K., Manning T. C., Holler P. D., Roy E. J., Kranz D. M. (1999) Bispecific agents target endogenous murine T cells against human tumor xenografts. Int. J. Cancer 83, 141–149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.