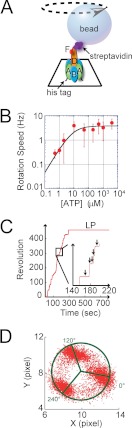
FIGURE 1.
Two types of pauses found in rotation assay of V1-ATPase. A, experimental setup for the single-molecule experiment (not to scale). A single molecule of V1 was attached to a glass surface through His10 tags at each A subunit. A streptavidin-coated magnetic bead with a diameter of ∼500 nm was attached to the D subunit that, along with the F subunit, constitutes the rotary shaft. V1 rotates counterclockwise. B, rotational rate versus [ATP]. By fitting with Michaelis-Menten equation, the maximum rate (Vmax) and Michaelis-Menten constant (Km) were determined as 3.8 rev/s and 8.1 μm. The apparent rate constant of ATP binding (konATP) was estimated as 1.39 × 10−6 s−1 m1 from Vmax/Km. C, rotation trajectory of a single V1 molecule. The molecule showed frequent reversible pauses, which we called the short pause. After ∼5 min of rotation, the molecule lapsed into an irreversible long pause state, from which spontaneous recovery was never observed unless forcibly rotated. D, X-Y trajectory of the molecule shown in C before lapsing into the LP state. The pausing positions of the SP states were separated by 120° intervals.