FIGURE 2.
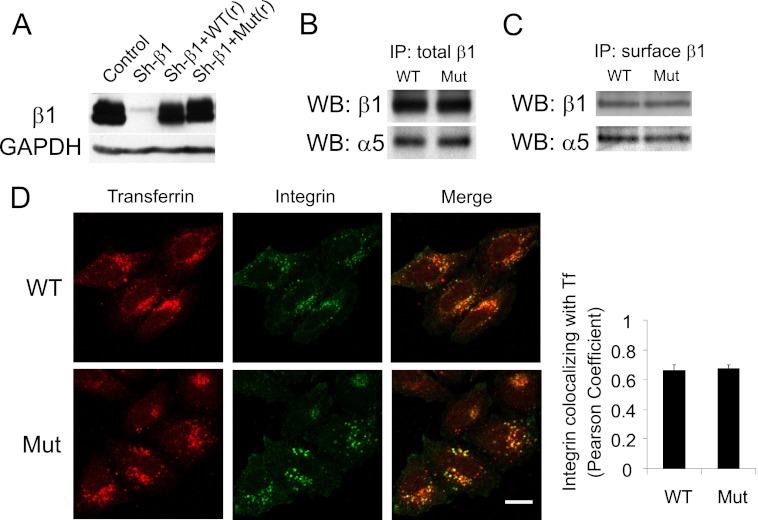
Replacing endogenous β1 with a β1 mutant that cannot bind efficiently to ACAP1. A, cell lines stably expressing different forms of integrin β1. The lentiviral system was used to replace endogenous β1 with transfected forms, which involved stably expressing shRNA against integrin β1 (Sh-β1), followed by stable expression of wild-type or mutant β1, which was resistant to shRNA through the introduced silent mutations. Expression was assessed by immunoblotting of whole cell lysates, with the level of GAPDH serving as a loading control. B, mutant β1 (Mut) assembles with α5 similarly as wild-type β1. Stable cell lines as described above were lysed, followed by immunoprecipitation (IP) for β1 and then immunoblotting for proteins as indicated. WB, Western blot. C, integrin heterodimers with mutant β1 are expressed at similar levels on the cell surface as those with wild-type β1. Stable cell lines as described above were bound with anti-β1 antibody at the cell surface. This pool of surface β1 was then immunoprecipitated, followed by immunoblotting for proteins as indicated. D, the mutant and wild-type integrins accumulate similarly at the recycling endosome under basal conditions. After allowing the surface pools of integrin and transferrin (Tf) to internalize for 2 h under basal conditions, cells were assessed by confocal microscopy comparing the distribution of integrin (green) and transferrin (red). Scale bar = 15 μm. The graph shows quantitative colocalization analysis, which reveals that wild-type and mutant integrins accumulate to a similar degree with internalized transferrin. The mean ± S.E. from three experiments is shown.