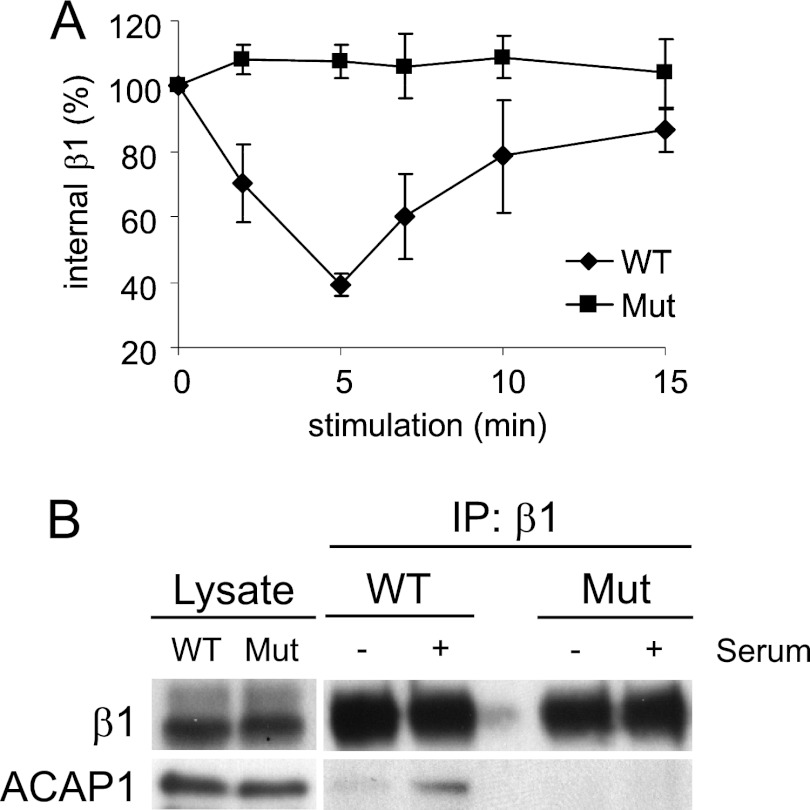
FIGURE 3.
Mutant β1 that cannot bind efficiently to ACAP1 also cannot recycle efficiently. A, integrin β1 with a mutation (Mut) of residues critical for its direct binding to ACAP1 shows a reduced ability to recycle. A lentiviral system was used to deplete endogenous β1, followed by stable expression of transfected forms as indicated. Surface β1 integrins (tracked by antibody binding) were then allowed to accumulate at the recycling endosome under basal conditions, followed by stimulation at the times indicated for their recycling. The mean ± S. E. from three experiments is shown. B, stimulation-dependent association of endosomal β1 with ACAP1 is reduced by mutations in β1 that reduce its binding to ACAP1. Endosomal β1, tracked as described above, was assessed for association with ACAP1 by co-precipitation. IP, immunoprecipitation.