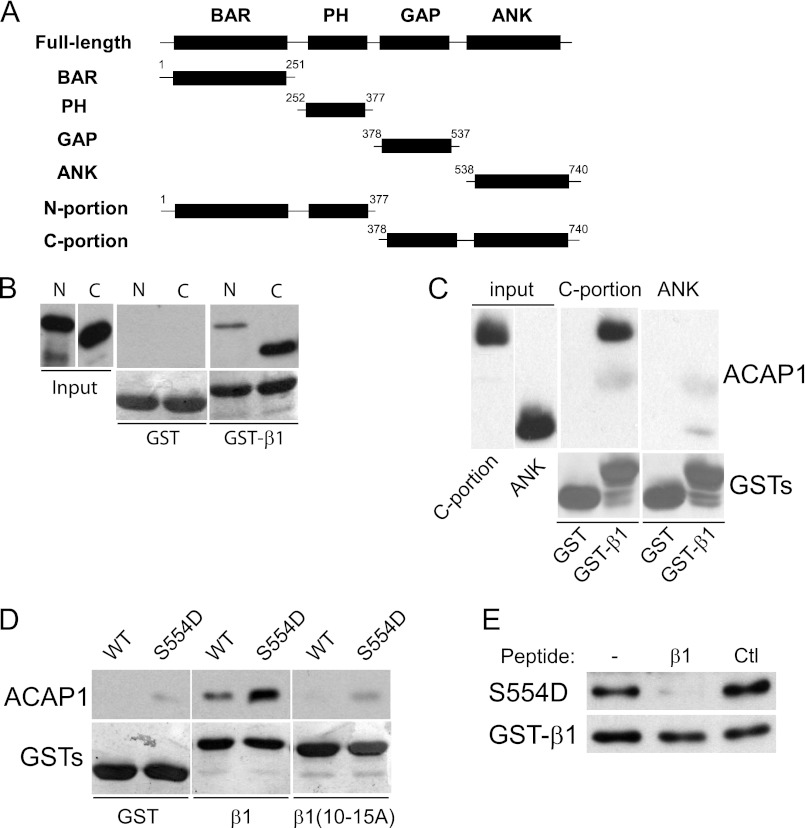
FIGURE 4.
C-terminal portion of ACAP1 reproduces regulated cargo binding. A, schematic showing different domain constructs of ACAP1 generated as recombinant proteins. B, binding to the cytoplasmic domain of integrin β1 by different portions of ACAP1. The cytoplasmic domain of β1 as GST fusions bound to beads was incubated with the N- or C-terminal portion of ACAP1 in pulldown experiments. C, the ANK domain has reduced ability in binding to the β1 cargo. Recombinant forms of ACAP1 as indicated were incubated with GST-β1 on beads in pulldown experiments. D, regulation of cargo binding by Ser-554 is reproduced by the C-terminal portion. Different GST fusions on beads as indicated were incubated with the C-terminal portion of ACAP1 (WT or mutant S554D) in pulldown experiments. WB, Western blot. E, the recycling sorting signal in β1 as a free peptide competes for binding of the mutant (S554D) C-terminal portion to GST-β1 on beads. The pulldown experiment was performed. A free peptide containing an irrelevant sequence of similar length (derived from the cytoplasmic domain of Wbp1) was used as control (Ctl).