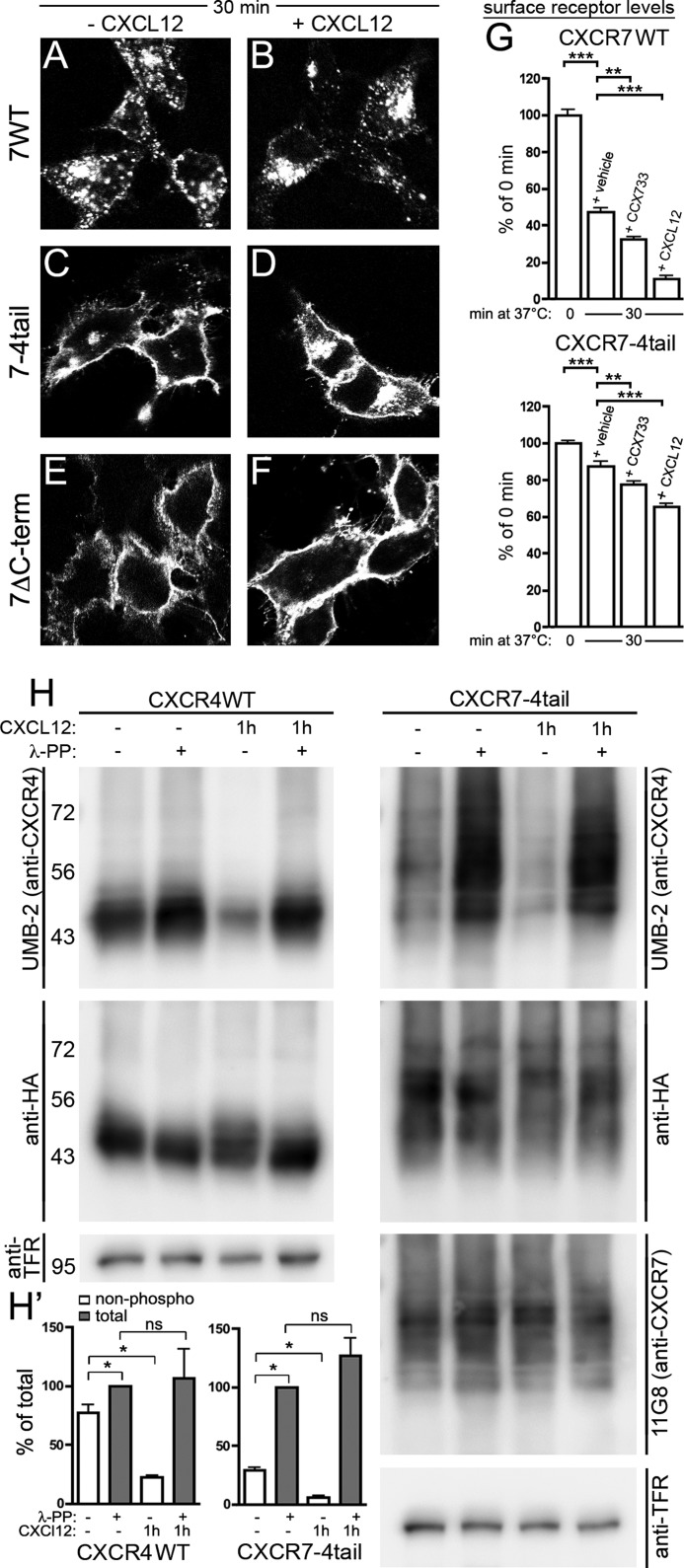
FIGURE 5.
Ligand-dependent activation and internalization of the CXCR7–4tail mutant. HEK293 cells were transiently transfected with CXCR7-WT, CXCR7–4tail, CXCR7ΔC-term, or CXCR4-WT as indicated. A–G, surface receptors were pulse labeled with anti-HA (A–D and G) or 11G8 anti-CXCR7 (E and F) antibody at 4 °C. A–F, confocal images show the subcellular receptor localization after 30 min at 37 °C in the absence (A, C, and E) or presence of CXCL12 (B, D, and F). CXCR7–4tail undergoes only ligand-dependent internalization (C and D), whereas CXCR7ΔC-term is internalization defective (E and F) and CXCR7-WT internalizes ligand independently (A and B). G, quantitative analysis of ligand-induced receptor internalization by ELISA. Cultures were incubated for 30 min at 37 °C with vehicle, CCX733 or CXCL12. Surface receptor levels are given as percentage of the surface receptor level immediately after pulse labeling (0 min). Results represent mean ± S.E. calculated from three to four independent experiments with 4 repeats each. Note that CCX733 and CXCL12 significantly increase receptor internalization. Asterisks indicate differences between the indicated groups (one-way ANOVA). H, immunoblots of wheat germ lectin-agarose (WGA)-purified cell lysates. Cultures were stimulated with CXCL12 and lysates were dephosphorylated with λ-PP as indicated. Upper panel, detection of CXCR4-WT and CXCR7–4tail protein using the anti-CXCR4 antibody UMB-2, which recognizes only the nonphosphorylated C-terminal CXCR4 epitope. Comparison of λ-PP-treated and λ-PP-untreated samples of nonstimulated cultures (lanes 1 and 2) indicates little constitutive phosphorylation of the UMB-2 epitope in CXCR4-WT but strong constitutive phosphorylation in CXCR7–4tail. CXCL12 treatment causes almost complete phosphorylation of the UMB-2 epitope both in CXCR4-WT and CXCR7–4tail (lanes 3 and 4). Middle and lower panels, aliquots of the samples shown in the upper panel were detected with anti-HA, 11G8, and anti-transferrin receptor (TFR) antibodies as indicated to confirm equal protein loading. H′, quantitative analyses of UMB-2 signal intensities in immunoblots from CXCR4-WT and CXCR7–4tail-transfected HEK293 cells using CCD camera-based densitometry. Relative amounts of nonphosphorylated (non-phospho) and total receptors were determined by measuring the UMB-2/HA ratios in λ-PP-untreated and λ-PP-treated samples, respectively. Results from 3 independent experiments were averaged and expressed as percentage of total receptor in cultures not receiving CXCL12 (*, p < 0.05, paired Student's t test).