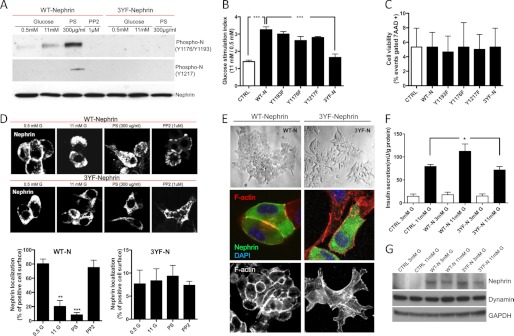
FIGURE 1.
Nephrin phosphorylation affects responsiveness to glucose and cell morphology. A, the degree of Nephrin phosphorylation at tyrosine residues 1176/1193 and 1217 was studied by WB in MIN6-C3 subclones (glucose unresponsive) overexpressing WT-Nephrin or 3YF-Nephrin. The cells were stimulated with glucose (11 mm), with PS (300 μg/ml) or PP2 (1 μm) in combination with 11 mm glucose. Although both glucose and PS induced phosphorylation of WT-Nephrin, PS induced phosphorylation of Nephrin only at residue Tyr-1217. PP2 and transfection with 3YF-Nephrin prevented Nephrin phosphorylation. B, static incubation experiments were performed in MIN6-C3 cells transfected with an empty vector (CTRL), WT-Nephrin (WT-N), single tyrosine residues mutants (Y1193F, Y1176F, and Y1217F), or 3YF-Nephrin (3YF-N) and demonstrated that Nephrin phosphorylation is essential for GSIR. ***, p < 0.001, n = 6. C, bar graph analysis of cell viability (% of 7-amino-actinomycin D (7AAD) positive cells) in MIN6-C3 cells transfected with an empty vector (CTRL), WT-Nephrin (WT-N), single tyrosine residues mutants (Y1193F, Y1176F, and Y1217F), or 3YF-Nephrin (3YF-N). No effects on cell viability were observed. D, Nephrin trafficking after 11 mm glucose, PS (300 μg/ml), and PP2 (1 μm) stimulation was studied in WT-Nephrin or 3YF-Nephrin-overexpressing cells. Although glucose and PS induced WT-Nephrin internalization, PP2 treatment resulted in a peripheral WT-Nephrin distribution. On the contrary, 3YF-Nephrin was localized in the intracellular compartments and was not affected by glucose, PS, or PP2. Bar graph analyses of the co-localization of WT-Nephrin and 3YF-Nephrin with the FM dye 4-64 utilized to stain plasma membranes are also shown. **, p < 0.01; ***, p < 0.001. E, WT-Nephrin-overexpressing cells presented with a normal morphology by light microscopy, whereas 3YF-Nephrin-overexpressing cells seemed more scattered and were characterized by impaired cell to cell contacts. Confocal images of WT-Nephrin-overexpressing cells showing a localization of WT-Nephrin primarily on the cell surface and at sites of cell to cell contact, whereas 3YF-Nephrin seemed to be primarily localized to intracellular compartments. 3YF-Nephrin-overexpressing cells did not show any cell to cell contacts. Phalloidin staining (bottom) was performed to demonstrate that cortical F-actin distribution seen in WT-Nephrin-overexpressing cells was impaired in 3YF-Nephrin-overexpressing cells. Three independent experiments with imaging of 5–10 cells each were performed. F, bar graph analysis for insulin secretion in human islets isolated from four different donors transfected with an empty vector (control, CTRL), WT-Nephrin (WT-N), or 3YF-Nephrin (3YF-N) exposed to either 3 or 11 mm glucose. *, p < 0.05. G, representative Western blot demonstrating efficiency of transfection of WT-Nephrin and 3YF-Nephrin in human islets.