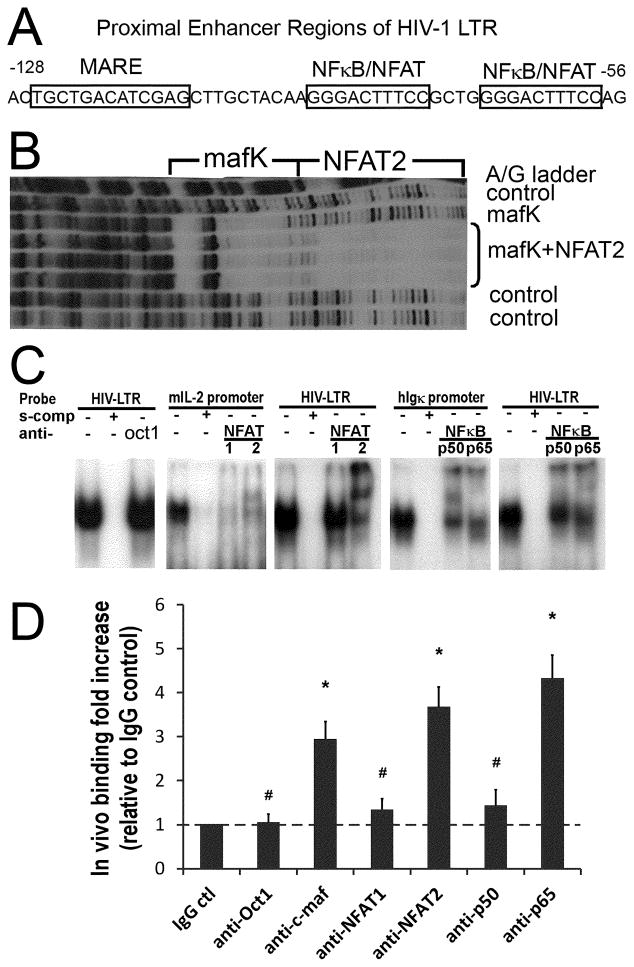
Figure 4. c-maf, NFAT2, and NFκB p65 preferentially bind the proximal HIV-1 LTR.
(A) The dual NFκB/NFAT binding sites and the predicted c-maf binding site (MARE) in the proximal HIV-1 LTR are depicted. (B) The in vitro footprint of the HIV-1 LTR DNase I digestion shows a mafK induced footprint of the LTR just upstream and adjacent to the NFAT2 footprint. (C) Nuclear extracts prepared from activated IL-4 primed human CD4 T cells bind the HIV-1 LTR NFκB/NFAT dual site probe (panel 1, 3, 5), the mouse proximal IL-2 promoter NFAT site (panel 2), and the human Igκ promoter NFκB site (panel 4) in gel shift assays (see Materials and Methods for details). NFAT1 and NFAT2 bind the IL-2 promoter similarly (panel 2), whereas, NFAT2 preferentially binds over NFAT1 to the HIV-1 proximal LTR (panel 3). Likewise, NFκB p65 and p50 bind the Igκ promoter NFκB site similarly (panel 4), but NFκB p65 binds preferentially, relative to NFκB p50, to the proximal HIV-1 LTR (panel 5). Oct1 protein acts as a control that does not bind to the proximal HIV-1 LTR (panel 1). Results are representative of 3 independent experiments. (D) Real-Time PCR was performed to quantify transcription factors bound to the integrated HIV-1 LTR in vivo as detected by ChIP. The results reveal c-maf, and NFAT2 over NFAT1, and NFκB p65 over p50, preferentially bind to the HIV-1 LTR in vivo. Rabbit IgG and anti-Oct1 antibodies are negative controls. The relative fold increases of transcription factor binding are calculated in comparison with the rabbit IgG control. # p>0.05 when comparing with IgG control; * p<0.05 when comparing with IgG control; n=5.