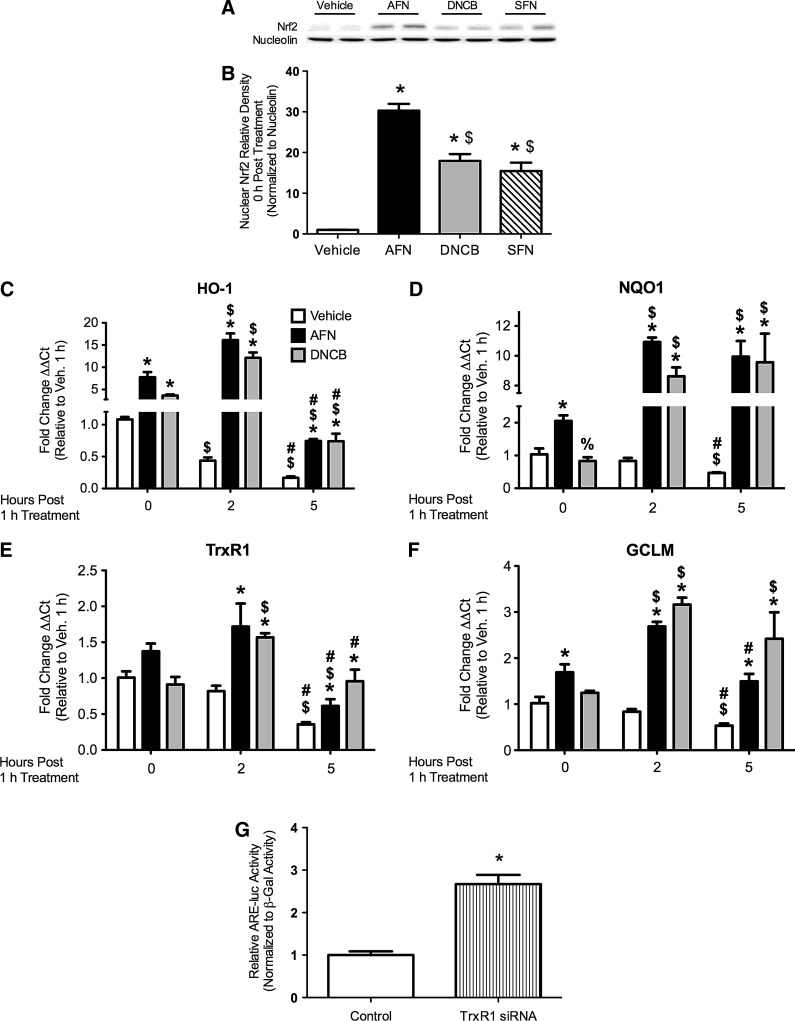
FIG. 2.
Nrf2 nuclear abundance and ARE-driven mRNA levels in mtCCs after AFN, DNCB, or SFN treatment and ARE-luciferase activity in mtCCs treated with TrxR1-specific siRNA. (A) Representative Western blot. (B) AFN, DNCB, and SFN treatment increased nuclear Nrf2 abundance by 30-fold, 18-fold, and 15-fold, respectively, compared with vehicle-treated controls. Data (mean±SEM, n=5–8) were normalized to nucleolin and analyzed by one-way ANOVA with Bonferroni post hoc with significance noted at p<0.05. (*different than vehicle; $different than AFN). (C–F) mtCCs were treated with 1 μM AFN or 15 μM DNCB for 1 h. qRT-PCR analyses indicated greater levels of (C) HO-1, (D) NQO1, (E) TrxR1, and (F) GCLM after AFN and DNCB treatment than in vehicle-treated controls. Analyses indicated the independent effects of AFN and DNCB and an interaction between AFN/time and DNCB/time for HO-1, NQO1, TrxR1, and GCLM. dCt values (mean±SEM, n=3) were analyzed by two-way ANOVA with Bonferroni post hoc with significance noted at p<0.05. (*different than same time/vehicle;%different than same time/AFN; $different than same treatment/0 h; #different than same treatment/5 h). (G) mtCC were transfected with TrxR1-specific siRNA or empty vector control, ARE-luciferase, and respiratory syncytial virus-β-galactosidase plasmid DNA for 48 h. Luciferase activity was 2.7-fold greater in the TrxR1 siRNA-treated cells than in empty vector-treated controls. Data (mean±SEM, n=9) were normalized to β-galactosidase and analyzed by an unpaired t-test with significance noted at p<0.05 (*different than empty vector control). ARE, antioxidant response element; GCLM, glutamate-cysteine ligase modifier subunit; HO-1, heme oxygenase 1; NQO1, NADPH:quinone oxidoreductase 1; Nrf2, nuclear factor E2-related factor 2; qRT-PCR=quantitative reverse transcriptase–polymerase chain reaction.