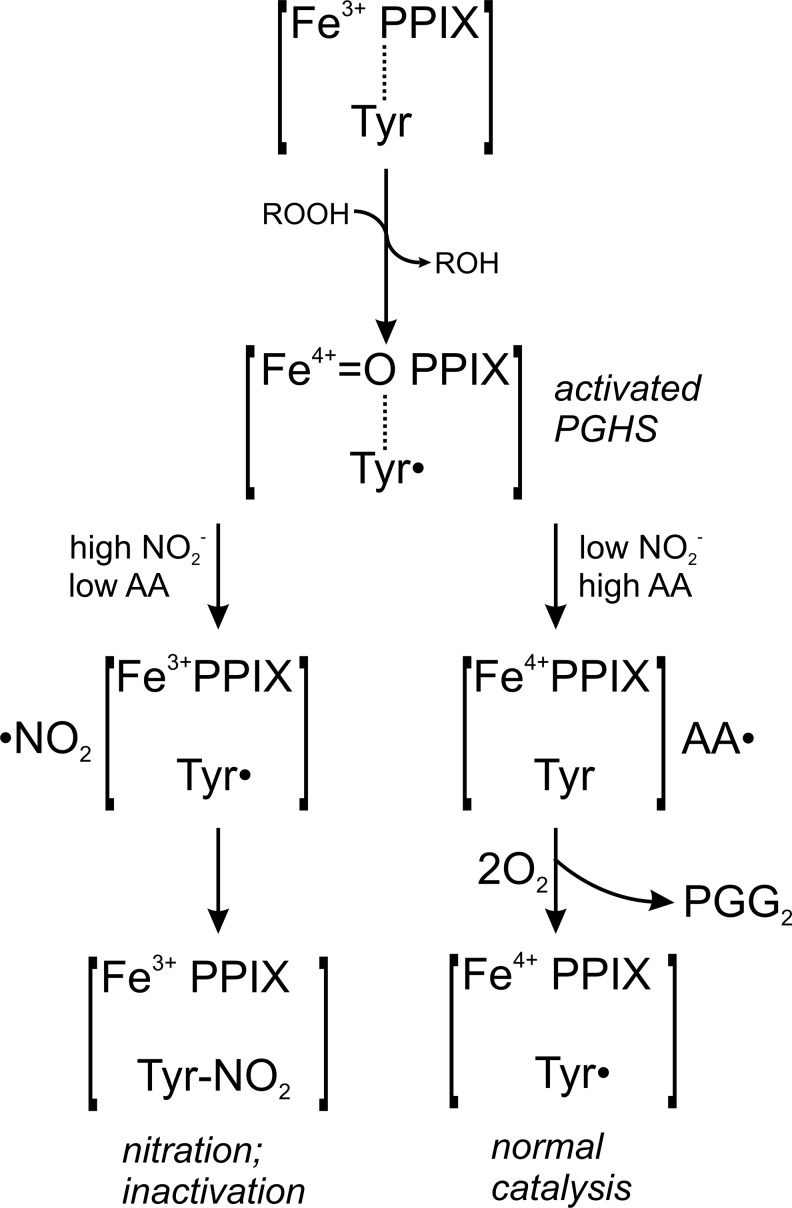
FIG. 8.
Inhibition of PGHS by autocatalytic activation of NO2−. The prosthetic ferric heme [Fe3+ PPIX] of the peroxidase domain is oxidized by peroxides (ROOH) to an unstable radical cation intermediate [Fe4+=O PPIX•+] (9, 37, 42). It is assumed that an equilibrium between the ferryl porphyrin radical cation and the ferryl-tyrosyl state [Fe4+=O PPIX]+[Tyr•] exists. Interaction of NO2− with the radical cation formed at the peroxidase site would lead to the formation of a •NO2 radical and leaves the prosthetic heme in its ferryl form that would be oxidized by the presence of peroxides. In the presence of low levels of substrate AA (left), the newly generated •NO2 radical could then reach the tyrosyl radical at the cyclooxygenase active site either by diffusion via the main cyclooxygenase channel, or by one of the four alternative smaller channels connecting the active site with the exterior, leading to the nitration (Tyr-NO2) and inactivation of PGHS. In the presence of low NO2− concentrations, the tyrosyl radical would interact with AA to form AA• and ultimately 15-hydroperoxy prostaglandin-9,11,-endoperoxide (PGG2) (right), according to the proposed concept by Ruf and Kulmacz et al. (9, 20, 21, 37, 40, 42). Under these circumstances, PGG2 is reduced into PGH2 by the peroxidase site with electrons originating from the active site tyrosyl residue, leaving a Tyr• radical ready for the initiation of a new catalytic cycle. PPIX, protoporphyrin IX. PGG2, 15-hydroperoxy prostoglandin-9,11,-endoperoxide; PGH2, 15-hydroxy prostaglandin-9,11,-endoperoxide.