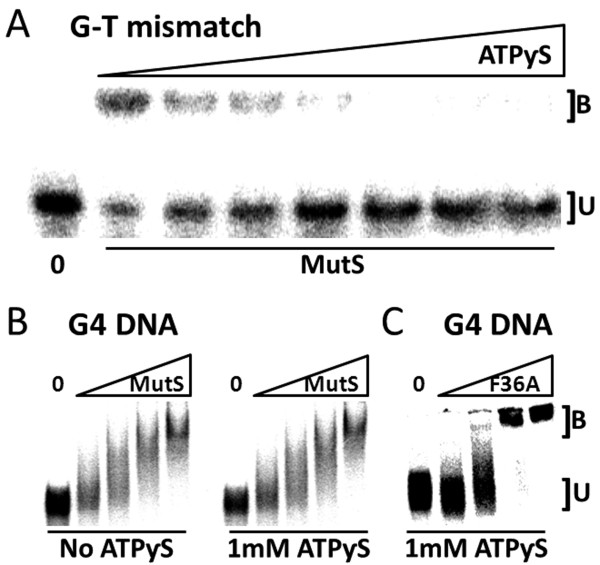
Figure 4 .
MutS association with ATP induces the release of G-T mismatches but not G4. (A) MutS dissociates from mismatches in presence of ATPγS. Representative mobility shift assay with radiolabeled G-T mismatch substrate and 60 nM WT-MutS per lane (excluding Lane 1 “0”, no WT-MutS control). Lane 2 contained no ATPγS (positive control) and lanes 3–8 contained increasing concentrations of ATPγS from 31 μM to 1.0 mM generated by a 1:1 serial dilution. B, oligonucleotide bound by MutS; U, unbound. (B) MutS binds G4 in presence of ATPγS. Representative mobility shift showing G4 association with WT-MutS with or without ATPγS. Lanes 1 and 6 contain no protein while lanes 2–5 and 7–10 contain increasing amounts of WT-MutS (19, 38, 75, and 150 nM). (C) Mobility shift evaluating G4 binding in the presence of 1 mM ATPγS as in B except F36A-MutS is examined in place of WT-MutS. Data (Additional file 4) represent the mean of three independent experiments with standard error.