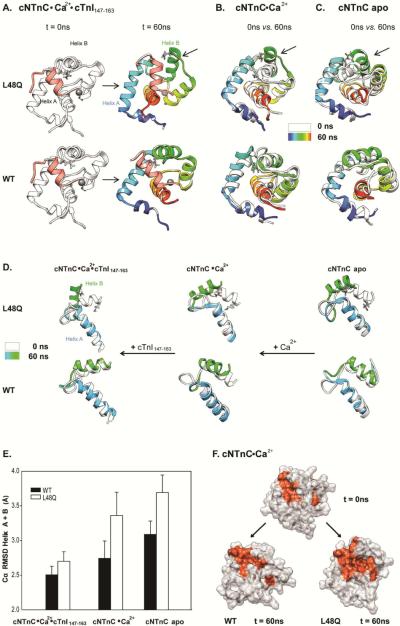
FIGURE 9.
Effects of L48Q on the mobility of helix B in cNTnC. A. Snapshots from cNTnC(L48Q)°Ca2+°cTnI147-163 and cNTnC°Ca2+°cTnI147-163 simulations at 0ns(white) and 60ns(rainbow). B. Comparisons of 0ns (white) vs. 60ns (rainbow) Ca2+ saturated structures for cNTnC(L48Q)°Ca2+ (upper) and cNTnC°Ca2+. C. Comparisons of 0ns (white) vs. 60ns (rainbow) apo state structures for cNTnC(L48Q) (upper)and cNTnC. Residues L48 and Q48 are shown as sticks. D. Helices A and B truncated from structures at 0 ns (white) and 60 ns (A helix: blue and B helix: green) for NTnC(L48Q)°Ca2+°cTnI147-163 (left),cNTnC(L48Q) °Ca2+ (middle), and cNTnC°Ca2+ (right). The helices are shown rotated 90° right from the structures in panels A–C to better view the A/B helix angle. E. Cα RMSD for helices A and B of L48Q (white) and WT (black) averaged over all simulations of cNTnC°Ca2+°cTnI147-163, cNTnC°Ca2+ and cNTnC apo. F. Surface rendering of cNTnC°Ca2+ for structures from the WT (0 ns, upper; and 60 ns,lower left) and L48Q (60 ns, lower right) simulations. The hydrophobic residues (F20, A23, F24, I26, F27, I36, L41, V44, L48, L57, M60, F77, M80, M81) are colored red with the rest of the protein in white.