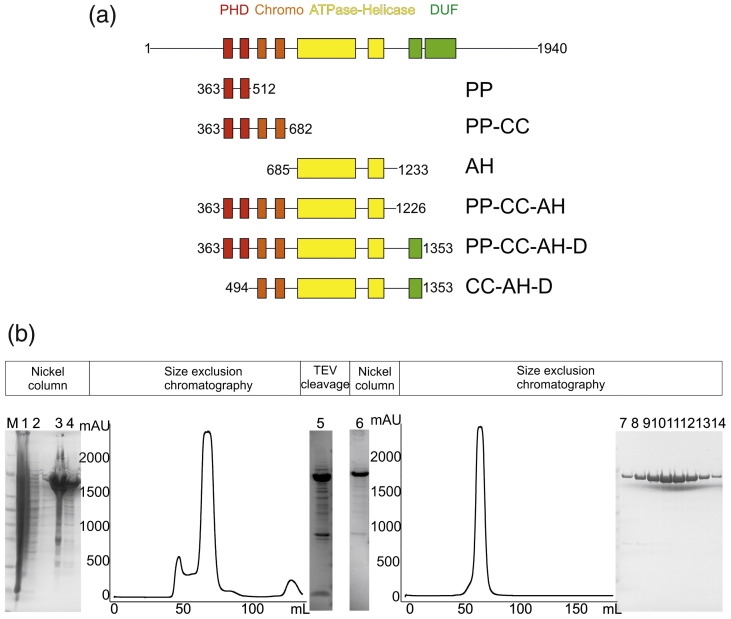
Fig. 1.
Recombinant CHD4 protein production and characterization. (a) Human CHD4 protein constructs. PHD (P) domains are colored red, chromo (C) domains are colored orange, the ATPase-helicase (AH) domain is colored yellow, and the domains of unknown function (D) are colored green. (b) Purification strategy for CHD4 constructs. Here, SDS-PAGE gels are presented following the nickel affinity purification, TEV protease cleavage, and size-exclusion chromatography steps applied to construct CC-AH-D. Similar strategies were followed for the other CHD4 constructs. Lane 1, total lysate; lanes 2–4, the elutions from nickel beads using buffer supplemented with 10, 30, and 300 mM imidazole, respectively; lane 5, TEV-cleaved protein; lane 6, flow-through following reapplication of TEV-cleaved protein to a second nickel resin column; lanes 7–14, fractions from a peak with an elution volume corresponding to the predicted molecular mass of CC-AH-D, eluted from HiLoad Superdex 200 16/60 Prep Grade filtration column. Protein samples were resolved on a NuPAGE 4–12% Bistris gel stained with Coomassie Brilliant Blue stain.