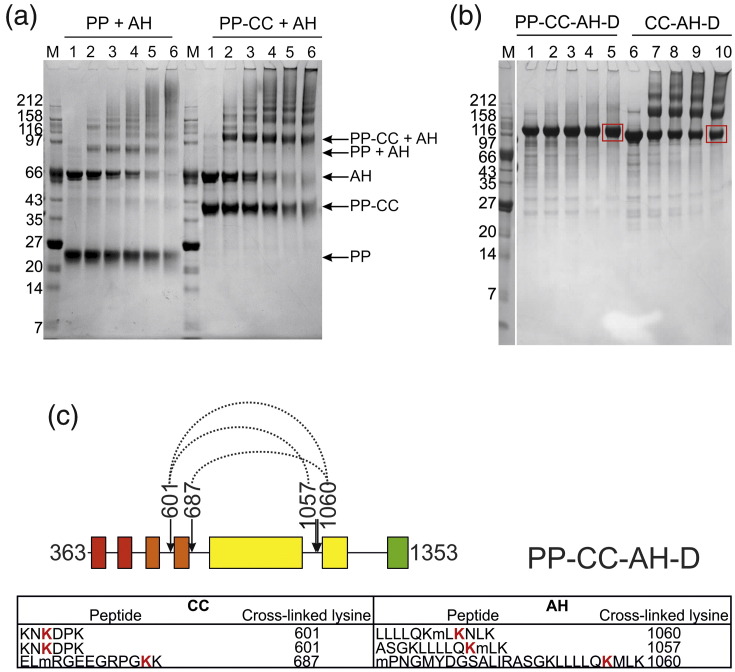
Fig. 4.
Interdomain cross-links in CHD4. (a) The ATPase domain of CHD4 (AH) was mixed with either the tandem PHD domains (PP) or the tandem PHD plus tandem chromo domains (PP-CC) at a 1:1 molar ratio, and was then incubated with H12/D12-labeled BS3 cross-linker in the following protein:cross-linker molar ratios: 1:0 (lane 1), 1:20 (lane 2), 1:50 (lane 3), 1:100 (lane 4), 1:200 (lane 5), and 1:500 (lane 6) for 30 min at room temperature. The protein molecular mass marker (M) is NEB Broad Range. Each reaction was stopped by the addition of 1/10 volume of 1 M Tris, pH 8.0, for 15 min. The reaction mix was separated on a NuPAGE 4–12% Bistris gel and stained with Coomassie Brilliant Blue stain. (b) The above procedure was repeated for constructs PP-CC-AH-D and CC-AH-D separately, each at the following protein:cross-linker ratios: 1:0 (lanes 1 and 6), 1:20 (lanes 2 and 7), 1:50 (lanes 3 and 8), 1:100 (lanes 4 and 9), and 1:200 (lanes 5 and 10). SDS-PAGE bands from lanes 5 and 10 found to have a molecular mass consistent with that of either monomeric PP-CC-AH-D or monomeric CC-AH-D, respectively, are indicated in red boxes. (c) The indicated gel bands were excised, trypsinized, and subjected to LC–MS/MS analysis. Cross-links between the chromo and ATPase-helicase domains within each construct were identified using Xlink-Identifier and are indicated by dotted lines. The sequence of each cross-linked peptide is tabulated below, where each modified lysine is represented in bold, red typeface. The residue numbers of these modified sites are indicated within the table and above the figure. Oxidized methionine residues are denoted in lower case.