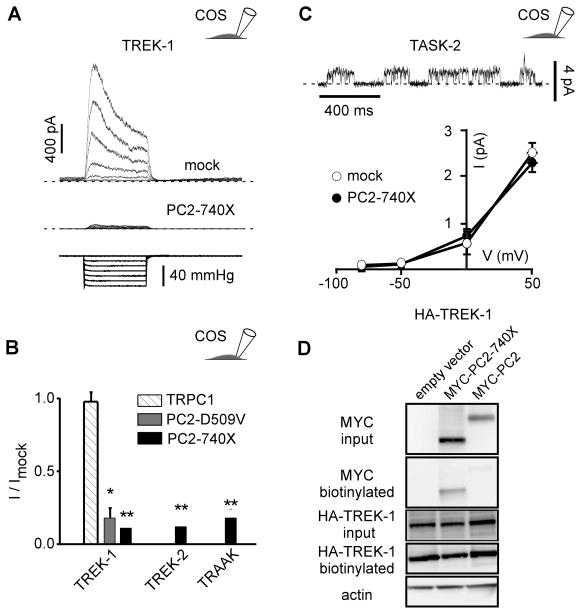
Figure 3. Stretch activation of TREK/TRAAK K2P channels expressed in COS cells is inhibited by PC2-740X.
A) TREK-1 currents (top trace) recorded in the cell-attached patch configuration at a holding potential of 0 mV in transiently transfected COS cells (co-transfected with a mock empty pIRES2-DsRed plasmid) in response to increasing negative pipette pressure from 0 to −60 mm Hg in steps of −10 mm Hg (bottom trace). When co-expressed with PC2-740 X, the amplitude of the stretch-activated current is dramatically reduced (middle trace). B) Mean current ratios (I/Imock) for TREK-1, TREK-2 or TRAAK at a holding potential of 0 mV in COS cells co-transfected with empty expression vector (n = 112), TRPC1 (n = 20), PC2-740X (n = 27, 27 and 22) or PC2-D509V (n = 28) in the cell attached patch configuration at a pressure of −60 mm Hg. C) TASK-2 currents in a transfected COS cell (together with a mock empty pIRES2-DsRed plasmid) recorded in the cell-attached patch configuration at a holding potential of 0 mV. I-V curves for TASK-2 in the absence (together with the empty expression vector; n = 9) or in the presence of PC2-740X (n = 6). D) Biotinylation experiments of TREK-1 in transfected COS cells. Neither PC2-740X nor WT PC2 affects expression of TREK-1 at the plasma membrane in COS cells. The three conditions tested are: HA-TREK 1 + empty vector; HA-TREK 1 + MYC-PC2-740X; HA-TREK 1 + MYC-PC2. Input blot and biotinylation blot were either probed with anti-MYC to detect PC2 or anti-HA to detect TREK-1. Blots were then stripped and probed with anti-actin. Only the input blot showed an actin signal which was equivalent for all 3 lanes. No detectable difference in TREK-1 expression at the cell surface was seen between any of these conditions. Data represent mean ± standard error of the mean.