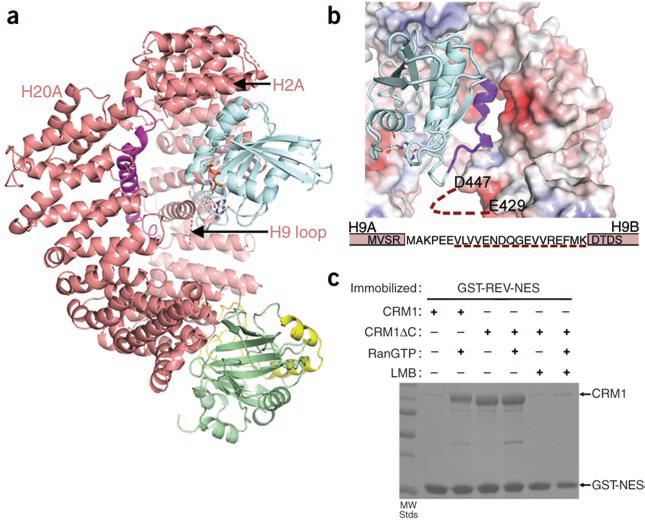
Figure 2.
Positive cooperativity of substrate and Ran binding to CRM1. (a) The CRM1–SNUPN binary intermediate can accommodate RanGTP. Repeats H2–H7 of SNUPN-bound CRM1 were superimposed with H2–H7 of the CSE1–Kap60– RanGTP complex10 (PDB 1WA5). CRM1 and SNUPN are colored as in Figure 1 and RanGTP is in blue. CSE1 and Kap60 are omitted for clarity. (b) The electrostatic surface potential of SNUPN-bound CRM1 is shown with RanGTP from the superimposed CSE1–Kap60–Ran complex. The basic patch of Ran (residues 129–142) is in purple, the H9 loop sequence is shown and a dashed line indicates the disordered portion of this loop. (c) Pulldown assays with immobilized GST-NES of HIV1-REV and CRM1 proteins in the presence or absence of RanGTP and/or leptomycin B (LMB) were visualized by SDS-PAGE and Coomassie blue staining.