Abstract
We recently described an immunocompetent Syrian hamster model for oncolytic adenoviruses (Ads) that permits virus replication in tumor cells as well as some normal tissues. This model allows exploration of interactions between the virus, tumor, normal organs, and host immune system that could not be examined in the immunodeficient or nonpermissive animal models previously used in the oncolytic Ad field. Here we asked whether the immune response to oncolytic Ad enhances or limits antitumor efficacy. We first determined that cyclophosphamide (CP) is a potent immunosuppressive agent in the Syrian hamster and that CP alone had no effect on tumor growth. Importantly, we found that the antitumor efficacy of oncolytic Ads was significantly enhanced in immunosuppressed animals. In animals that received virus therapy plus immunosuppression, significant differences were observed in tumor histology, and in many cases little viable tumor remained. Notably, we also determined that immunosuppression allowed intratumoral virus levels to remain elevated for prolonged periods. Although favorable tumor responses can be achieved in immunocompetent animals, the rate of virus clearance from the tumor may lead to varied antitumor efficacy. Immunosuppression, therefore, allows sustained Ad replication and oncolysis, which leads to substantially improved suppression of tumor growth.
Introduction
Oncolytic adenoviruses (Ads) have the potential to become valuable treatment modalities in the fight against cancer. Impressive suppression of tumor growth in animal models has been achieved, but this has not translated into consistent control of human cancer in clinical trials. The use of oncolytic Ad agents in clinical trials has demonstrated that they can be safely administered, with generally minimal toxicity, even when given intravenously.1–3 The limitation appears to be the attainment of consistent significant tumor responses. Perhaps the combination of bulky, advanced disease and relatively low vector doses used in clinical trials creates a scenario in which substantial antitumor efficacy is difficult to obtain. However, in the studies described here, we have achieved remarkable antitumor responses with relatively large tumor burden at treatment initiation.
We recently reported the development of a Syrian hamster model in which oncolytic Ad efficacy and safety can be evaluated in a permissive, immunocompetent animal.4 We found that most hamster tumor cell lines tested were quite permissive for Ad replication, which translated into oncolytic Ad efficacy against these cells when grown as tumors in hamsters. We also determined that Ad was capable of replication in hamster tumors as well as normal tissues such as the liver.
This permissive immunocompetent animal model provides the unique opportunity to investigate interactions between the virus, tumor, normal organs, and the host immune system that previously could not be examined in either immunodeficient or nonpermissive animal models used in the oncolytic Ad field. In particular, we were interested in evaluating the role of the immune system in oncolytic Ad therapy. We have previously shown that hamsters generate an anti-Ad immune response following intratumoral injection of oncolytic Ad.4 Although it might be assumed that virus oncolysis is the predominant mechanism of tumor suppression with oncolytic Ads that do not express immunomodulatory molecules, it is also possible that the immune response mounted against Ad or against the tumor itself mediates antitumor efficacy. It is also possible that the immune response limits efficacy by clearing the virus from the tumor. Certainly, efficacy has been achieved with oncolytic Ads in immunodeficient mice,5–9 but it is unclear how well this immunocompromised, nonpermissive model can predict the behavior of vectors when used in humans.
We sought to evaluate whether the immune system is instrumental or detrimental to oncolytic Ad efficacy by inducing immunosuppression with cyclophosphamide (CP). We chose a broad-spectrum immunosuppressive agent because this would allow us to evaluate the role of the immune system as a whole. CP is a potent immunosuppressive agent that is used in organ transplantation10–13 and is also used in the treatment of some malignancies such as leukemias, lymphomas, and certain solid tumors such as breast and ovarian cancer.14–16CP is a prodrug that requires metabolic activation in the liver to produce 4-hydroxycyclophosphamide. 4-Hydroxycyclophosphamide diffuses into cells, and because it is very unstable it spontaneously decomposes into the primary alkylating metabolite, phosphor-amide mustard.17 Phosphoramide mustard is responsible for DNA alkylation and apoptosis. Although CP could potentially inhibit Ad DNA replication, it has been shown that this only occurs at doses used in vitro that are ~50–100 times greater than the probable serum concentration of active drug.17,18
CP has previously been evaluated as an immunosuppressant in the context of replication-defective Ad gene therapy vectors to prolong transgene expression and allow multiple rounds of therapy. Immunosuppression with CP led to prolonged transgene expression and successful vector readministration in mouse models.19–21 Importantly, doses of CP similar to those used in our studies were shown to ablate cytotoxic T-lymphocyte and helper T-lymphocyte functions as well as to prevent the formation of Ad-neutralizing antibodies.19–21 Various other means of immunosuppression have also been evaluated in the context of oncolytic viruses.22–26
Results
Evaluation of CP immunosuppression in the hamster
We first evaluated the effects of CP in the Syrian hamster, such as the extent and duration of immunosuppression, the effect of CP on tumor growth, and whether the drug was tolerated for at least 4 weeks without significant toxicity. We found that CP caused a dramatic reduction in white blood cell counts, including lymphocytes, monocytes, neutrophils, and eosinophils, by 3 days after the first dose (data not shown). This reduction was sustained for the duration of the 4-week study. Histologically, CP caused moderate to marked lymphoid atrophy in the spleen, thymus, and lymph nodes. CP was well tolerated for the duration of the study, although anemia slowly developed. CP did not affect tumor growth compared with vehicle alone (P = 0.814; data not shown).
Assessment of oncolytic Ad efficacy in immunosuppressed animals
We next evaluated the effect of immunosuppression on the antitumor efficacy of oncolytic Ad vectors. Hamsters bearing large, established subcutaneous HaK adenocarcinomas were randomized into six treatment groups. Three groups began immunosuppressive therapy with CP, while the remaining three groups remained immunocompetent. The three groups within each arm of the study received different intratumoral therapies: VRX-007, wild-type Ad serotype 5 (Ad5), or vehicle. VRX-007 is an oncolytic Ad that overexpresses the Ad death protein,27 an Ad protein that promotes efficient virus release from infected cells.28,29 Intratumoral injections were initiated 4 days following immunosuppression, because the preceding study demonstrated that the effect of CP was evident by 3 days after the first dose.
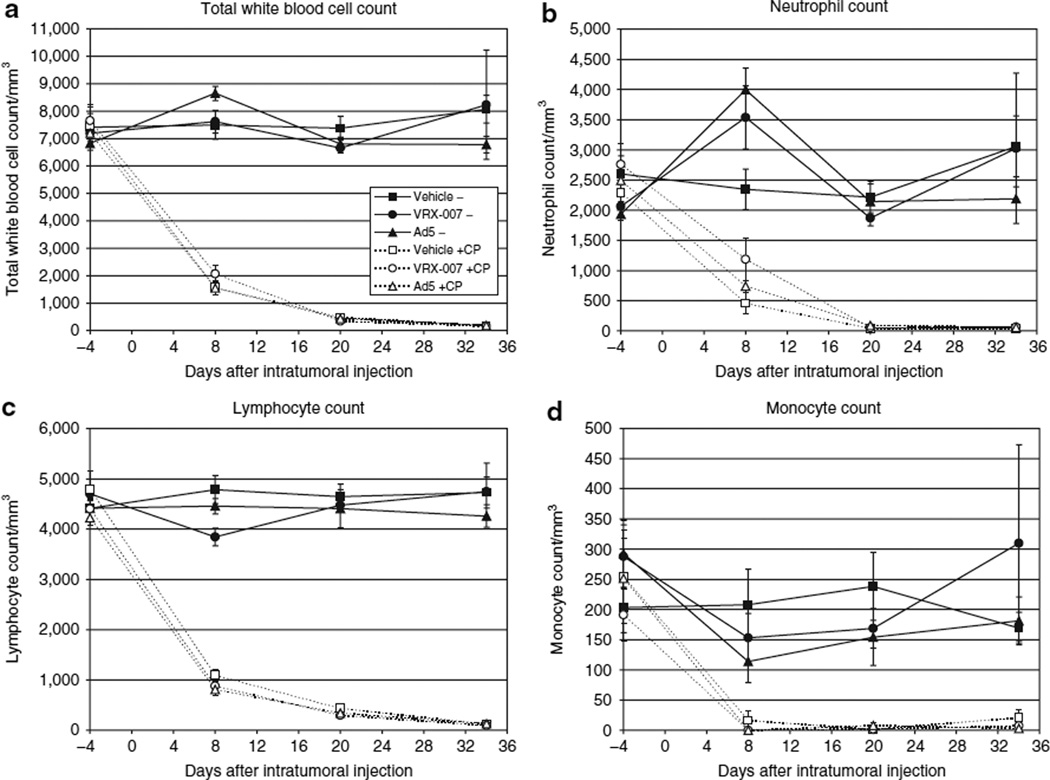
The state of immunosuppression was monitored during the course of this study by blood collection and hematology analysis at multiple time points. In animals that received CP, the total white blood cell counts had declined by the first blood collection time point (day 8) and they were suppressed for the study duration (Figure 1a). CP suppressed all leukocyte populations, including neutrophils (Figure 1b), lymphocytes (Figure 1c), monocytes (Figure 1d), and eosinophils (not shown). Importantly, immunosuppressed animals that were injected with virus did not show evidence of proliferation of any white blood cell type (cell counts were similar to those observed with immunosuppression alone). There are reports that lower CP doses preferentially deplete regulatory T cells.22,30 However, this is not the case for the doses used in this study considering the magnitude of suppression and that regulatory T cells constitute a minor fraction of the total leukocyte population.
Figure 1. Suppression of all immune cell types was observed in animals receiving cyclophosphamide (CP).
An antitumor efficacy study was conducted in which parallel groups of immunocompetent and immunosuppressed hamsters were treated with intratumoral injections of VRX-007, wild-type adenovirus serotype 5 (Ad5), or vehicle. In this study, the first dose of CP was given on day −4 and was followed by twice weekly CP dosing throughout the study. Intratumoral oncolytic Ad (or vehicle) injections were administered on days 0, 1, 2, 4–6. The tumor suppression data are shown in Figure 2. Blood was collected and hematological analysis was performed on days −4, 8, 20, and 34 to monitor immunosuppression. The counts of total white blood cells (a), neutrophils (b), lymphocytes (c), and monocytes (d) are shown. No basophils were observed. The data are shown as the mean of each treatment group, ±SEM, n = 6/group. The legend for all panels is shown in a.
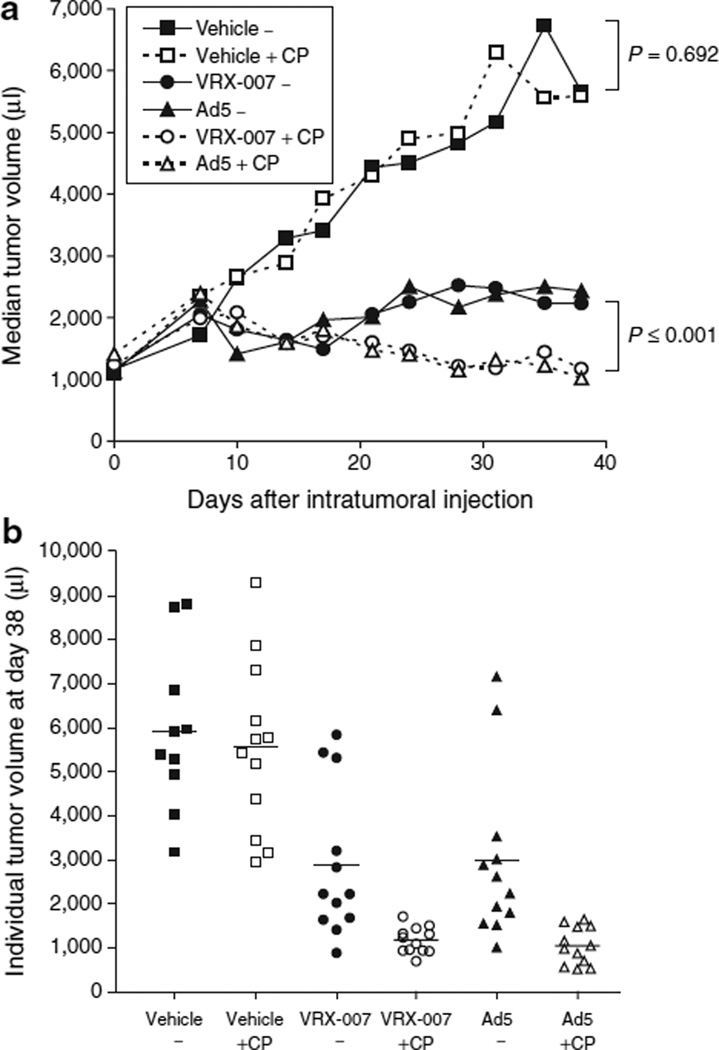
The efficacy of oncolytic Ad therapy was monitored by twice weekly measurement of tumor volumes. CP alone (Vehicle + CP) did not have a significant effect on tumor growth (Figure 2a, P = 0.692 versus “Vehicle−”). Immunocompetent hamsters that were given intratumoral injections of VRX-007 or Ad5 showed significant suppression of tumor growth relative to vehicle (Figure 2a, groups “VRX-007−” and “Ad5−”). However, given the large initial tumor burden of more than 1 ml, some of these tumors began to regrow at around 3–4 weeks. Remarkably, in immunosuppressed hamsters injected with VRX-007 or Ad5, tumor volumes steadily declined (Figure 2a, groups “VRX-007 + CP” and “Ad5 + CP”). The tumors in these animals reached maximum size at ~1 week, ranging from 1,500 to as large as 4,900 µl, and subsequently diminished in size in the following weeks. Oncolytic Ad was more effective in immunosuppressed animals (P = 0.001 for “VRX-007 + CP” versus “VRX-007” and P ≤ 0.001 for “Ad5 + CP” versus “Ad5−”).
Figure 2. Immunosuppression with cyclophosphamide (CP) resulted in significantly enhanced tumor control with oncolytic adenoviruses (Ads).
Following randomization based on tumor volume, animals in treatment groups receiving CP were administered the first dose of CP on day −4. CP was administered twice weekly for the duration of the study. The immunosuppression data are shown in Figure 1. Animals received intratumoral injections of VRX-007, Ad5, or vehicle on days 0, 1, 2, 4–6 (n = 12 for each of six groups). The mean tumor volume on day 0 was 1,294 µl. Tumor growth was monitored by tumor measurement with digital calipers. Due to anemia in animals treated with CP, the study was terminated at 41 and 42 days. (a) Median tumor volumes (in microliters) are shown for each group for the duration of the study. Statistically significant differences were found between virus treatment alone (“VRX-007−” or “Ad5−”) and virus plus CP (“VRX-007 + CP” or “Ad5 + CP”), in which all P ≤ 0.001. No significant difference was detected between “Vehicle−” and “Vehicle + CP” (P = 0.692). All groups that received intratumoral virus treatment showed statistically significant tumor suppression when compared with “Vehicle−” or “Vehicle + CP” (P ≤ 0.004 for all such comparisons). (b) Individual tumor volumes (in microliters) at the last tumor measurement, on day 38, are shown. Each point represents one animal and the bar represents the mean volume for each treatment group.
For the first 2 weeks, oncolytic Ad efficacy was similar in immunocompetent and immunosuppressed hamsters, but beginning around 3 weeks, some tumors in immunocompetent hamsters began to resume growth while tumors in immunosuppressed animals continued to decline in size. It is interesting to note the individual tumor sizes at study termination. In Figure 2bit is evident that immunosuppression allowed consistent and durable tumor suppression by oncolytic Ad, whereas tumor suppression was more variable and less effective in immunocompetent hamsters.
We also tested sera from hamsters in this study for the presence of Ad-neutralizing antibodies, as a functional measure of immunosuppression. We have previously shown that intratumoral injection of oncolytic Ads induces anti-Ad antibodies, including neutralizing antibodies.4Serum samples from immunocompetent hamsters that received intratumoral Ad therapy demonstrated neutralizing antibody titers from 1:1,280 to 1:5,120 at day 22 (Figure 3a) and from 1:5,120 to ≥1:20,480 at day 41–42 (Figure 3b). We did not detect Ad-neutralizing antibodies in sera from immunosuppressed hamsters at either time point. These findings are in concurrence with and are further supported by studies in which CP was found to suppress cytotoxic T lymphocyte, T-helper lymphocyte, and neutralizing antibody responses to replication-defective Ad.19–21
Figure 3. Adenovirus (Ad)-neutralizing antibody formation was inhibited by cyclophosphamide (CP).
Serum was obtained from half of the animals in the study (Figure 2) at day 22 and from all animals at study termination (days 41 and 42). The ability of these sera to neutralize adenovirus was quantitated in a cytopathic effect–based neutralization assay. The Ad-neutralizing antibody titer for each serum sample at (a) 22 days and at (b) 41–42 days is shown. Only a subset of vehicle-treated animals was tested. Serum samples that did not show inhibition of Ad infection at the lowest dilution tested (1:20) were considered to be negative.
Histopathology and immunohistochemistry of tumors in the antitumor efficacy study
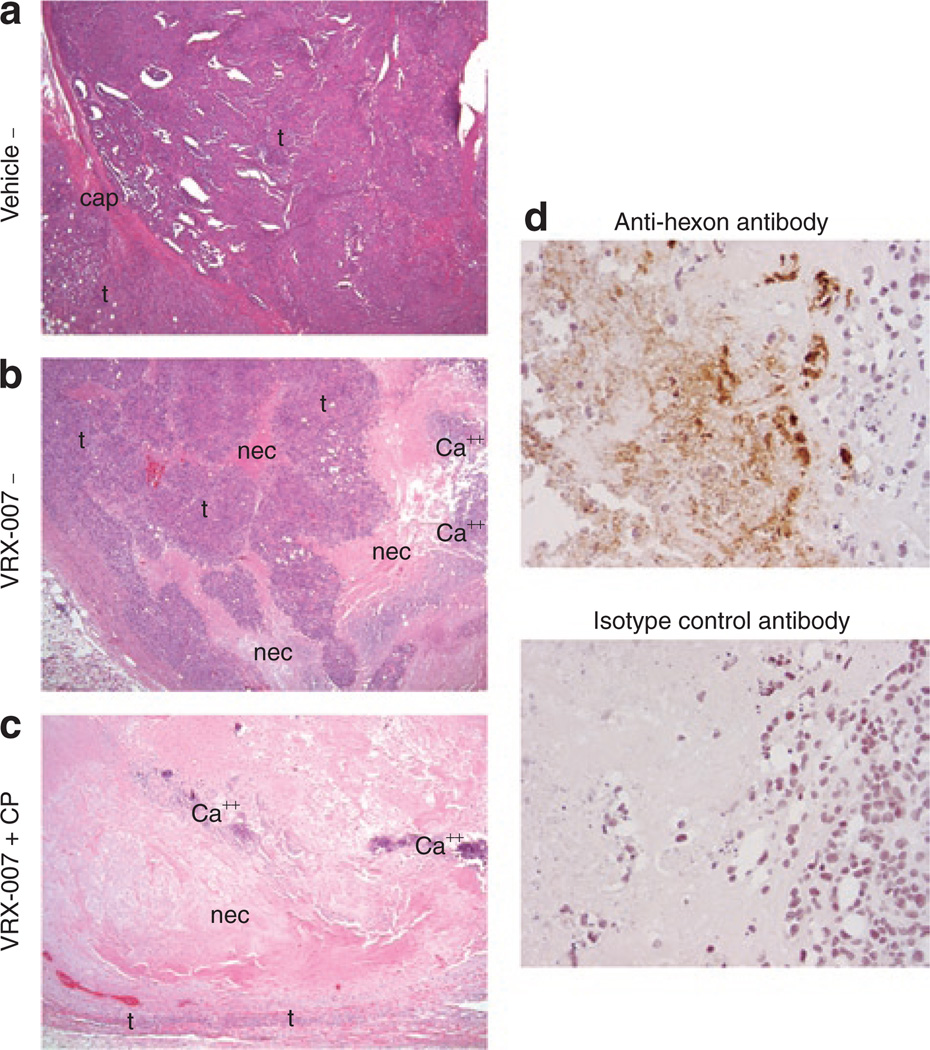
When tumors from this antitumor efficacy study were examined histologically at study termination, the effect of virus plus immunosuppression was even more striking. Figure 4a shows a typical vehicle-injected tumor in which there is minimal necrosis and the tumor architecture is well organized. In immunocompetent animals treated with oncolytic Ad, although some tumors had begun to regrow at study termination, many tumors demonstrated histological evidence of Ad antitumor effects. Tumors from these animals showed mild to moderate tumor disorganization and necrosis, with areas of decreased tumor cell density and many small islands of tumor cells (Figure 4b). The most remarkable changes in tumor histology were observed in animals that were both immunosuppressed and treated by oncolytic Ad. In these animals, tumor architecture was highly disorganized with moderate to severe confluent necrosis and calcification (an indicator of past necrosis) (Figure 4c). In many of these, much of the tumor mass that remained, and was being measured, was actually necrotic debris and calcification, and in several cases only a few islands of tumor cells remained.
Figure 4. Immunosuppression plus oncolytic adenovirus (Ad), VRX-007, resulted in striking changes in tumor histology and evidence of ongoing Ad infection by immunohistochemistry.
At the termination of the previously described study (Figure 2), hematoxylin and eosin (H&E)-stained tumor sections were examined histologically. (a) An H&E-stained tumor section of a representative animal in the “Vehicle−” group is shown, original magnification of ×10. This tumor is composed of viable tumor cells (designated by “t”) and virtually no necrosis. The tumor architecture is organized, with some gland formation, and the tumor cells are densely packed. A portion of the original tumor capsule (indicated by “cap”) is visible in this image. (b) Significant changes in tumor histology were noted in immunocompetent animals treated with oncolytic Ad, such as mild to moderate disorganization of tumor architecture, islands of live tumor cells (t) surrounded by necrosis (nec), calcification (Ca++), and decreased density of remaining tumor cells. A representative tumor from the “VRX-007−” group is shown, original magnification of ×10. (c) The most dramatic effect on tumors was noted in hamsters that were both immunosuppressed and treated with oncolytic Ad. In these animals, tumor architecture was disorganized, with moderate to severe necrosis (nec) and calcification (Ca++), as evidence of previous necrosis. In approximately half of these animals, only a small rim of viable tumor cells (t) remained and the majority of the mass was actually necrotic debris, as represented by the tumor from the “VRX-007 + CP” group shown here, original magnification of ×10. (d) Immunohistochemistry was performed with an anti-hexon (Ad structural protein) antibody on tumor sections obtained at study termination. Upper panel, only immunosuppressed animals treated with oncolytic Ad demonstrated positive anti-hexon staining nearly 6 weeks after the initial virus injection, as represented by the tumor shown from the “VRX-007 + CP” group. Lower panel, no staining was observed with an isotype control antibody on an adjacent tumor section from the same specimen. No areas of positive anti-hexon staining were observed at study termination in the tumors of immunocompetent animals treated with oncolytic Ad (data not shown). CP, cyclophosphamide.
Immunohistochemistry for the Ad hexon structural protein showed areas of ongoing virus infection in tumors of immunosuppressed animals (Figure 4d, upper panel) but not immunocompetent hamsters (data not shown) at study termination, nearly 6 weeks after initial virus injection. Staining was observed diffusely throughout necrotic areas as well as in the nuclei of infected tumor cells adjacent to necrotic areas. No staining was observed with an isotype control primary antibody (Figure 4d, lower panel).
Effect of immunosuppression on virus levels in tumors and other sites
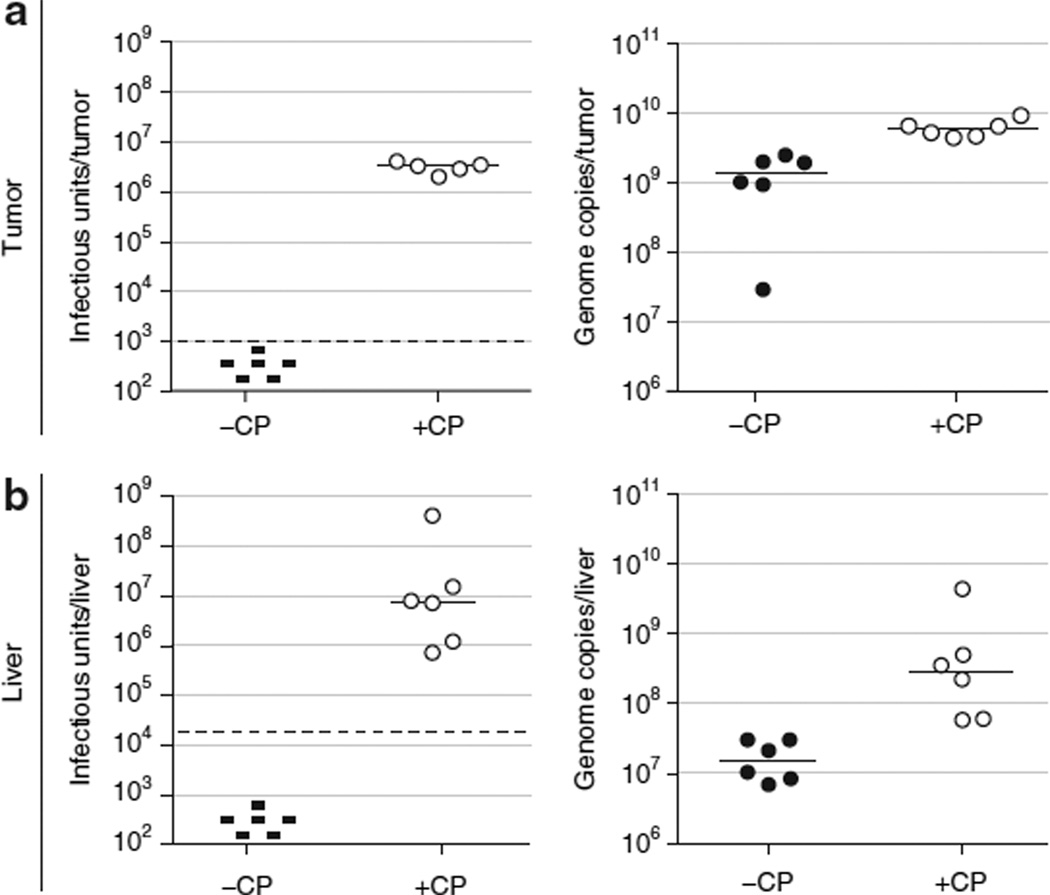
To address whether the increased antitumor efficacy of the vector observed in immunosuppressed hamsters was associated with higher levels of virus in the tumor, we determined the virus levels in the tumors of several animals from the efficacy study shown in Figures 1–4. We evaluated 12 animals treated with intratumoral VRX-007, 6 immunosuppressed and 6 immunocompetent. At the termination of this study (42 days after injection), no infectious virus was isolated from tumors of immunocompetent animals, while intratumoral virus levels in immunosuppressed animals were still quite elevated (Figure 5a, left panel). Similar data were obtained in the liver, in which infectious virus was recovered only from immunosuppressed animals (Figure 5b, left panel). Genome levels as determined by quantitative real-time PCR (Figure 5a and b, right panels) showed similar trends to infectious titers.
Figure 5. Immunosuppression allowed virus levels to remain elevated for the duration of the antitumor efficacy study, while virus was cleared in immunocompetent animals.
The tumor and a portion of the liver from 6 of 12 animals in the “VRX-007−” and “VRX-007 + CP” groups in the antitumor efficacy study (Figure 2) were obtained at study termination for virus quantitation. Tumor and liver samples were harvested and processed for virus titration by a tissue culture infectious dose 50 (TCID50) infectious assay as well as a quantitative real-time PCR assay for adenovirus (Ad) genomes. The titers obtained from (a) tumor and (b) liver specimens are shown. Left panels, TCID50 infectious titration. Right panels, quantitative real-time PCR titration. Each point represents the titer from one animal and the bars indicate the median for each group. The threshold for the TCID50 assay is shown by the dashed horizontal line. Samples in which no infectious virus was detected are indicated by “−.” CP, cyclophosphamide.
Liver and other organs were collected to evaluate whether any pathology was caused by CP directly or by virus shedding from tumors in immunosuppressed animals. Hepatocyte necrosis was not observed, suggesting lack of Ad-induced liver toxicity. We also evaluated potential liver toxicity due to Ad by examining levels of alanine transaminase and alkaline phosphatase in sera collected at 34 days after injection and found them to be normal in all treatment groups (data not shown). There were no significant differences in histological findings between animals that received vehicle and those that received either VRX-007 or Ad5. We did observe, however, a number of effects that were associated with CP. In some immunosuppressed animals, mild reduction in liver vacuolization, mild pigmentation of Kuppffer cells, and the presence of some karyomegalic cells (possibly megakaryocytes) in the liver were observed as an effect of CP (data not shown). We also confirmed that animals receiving CP demonstrated evidence of lymphoid atrophy in the spleen, lymph node, and thymus (data not shown). No other significant histological findings were observed in any other organs.
Time course analysis of vector replication in tumors, liver, and bloodstream in immunocompetent and immunosuppressed hamsters
To study the level of virus over time in immunocompetent and immunosuppressed hamsters, we performed an independent study. Hamsters were given a single intratumoral injection of VRX-007, Ad5, or vehicle. There were parallel groups of immunocompetent and immunosuppressed animals for each intratumoral treatment. We determined virus load in tumors, blood, and livers at 3, 9, and 22 days after injection.
Similar virus levels were present at 3 days in tumors of immunocompetent and immunosuppressed animals (Figure 6a, left panel). Virus levels in immunocompetent animals decreased by approximately tenfold by 9 days and by more than three orders of magnitude by 22 days. One immunocompetent animal at 9 days and two at 22 days showed <1 × 103 infectious units of virus (the lower limit of detection). In contrast, the amount of infectious virus in the tumors of immunosuppressed animals stayed elevated through 22 days. At 22 days, the difference in intratumoral virus quantity between immunocompetent and immunosuppressed hamsters was about three orders of magnitude. Titration of virus genome levels by real-time PCR showed similar trends (Figure 6a, right panel), with immunocompetent animals clearing the virus at later time points and immunosuppressed hamsters sustaining high levels of virus in the tumors. However, the difference in infectious virus levels between immunocompetent and immunosuppressed animals was more striking than the difference in viral genomes. Overall, these data demonstrate that while immunocompetent animals were able to clear the virus, immunosuppressed animals allowed continued virus replication within the tumor.
Figure 6. Adenovirus (Ad) levels in the tumor, blood, and liver of hamsters following a single intratumoral injection were determined in an independent replication kinetics study.
Animals received a single intratumoral injection of either VRX-007 (circle symbols) or wild-type Ad serotype 5 (Ad5) (triangle symbols). Parallel groups of immunocompetent (solid symbols) and immunosuppressed (open symbols) animals were evaluated at 3, 9, and 22 days after injection. At each time point, the tumor, blood, and liver were harvested and processed for virus quantitation by tissue culture infectious dose 50 (TCID50) infectious titration and quantitative real-time PCR titration. The titers obtained (a) per tumor, (b) per ml of blood, (c) and per liver samples are shown. Left panels, TCID50 infectious titration. Right panels, quantitative real-time PCR titration. Each point represents the titer from one animal and the bars indicate the median for each group. The threshold for each assay is shown by the dashed horizontal line. Samples in which infectious virus was detected but was below the threshold of the assay are indicated by “+”. Samples in which no infectious virus was detected are indicated by “−.”
We have previously shown that following intratumoral injection, Ad is detected in the blood and replicates within some normal tissues of the Syrian hamster, particularly the liver.4 We have now examined the level of virus in the blood and in the liver of immunocompetent and immunosuppressed hamsters. In immunocompetent hamsters, virus levels in the blood were highest at 3 days, with a median of 2.2 ×104 genome copies/ml of blood (Figure 6b, right panel) and <200 infectious particles/ml of blood (Figure 6b, left panel). At 9 and 22 days, genome levels declined and no infectious virus was recovered from immunocompetent animals. The amount of virus in the blood of immunosuppressed animals, on the other hand, remained at similar levels at all time points, with some animals at 9 and 22 days showing spikes of viremia in the range of 106 genome copies or 105 infectious virions per ml. In the liver, virus levels followed trends similar to what was observed in the blood. Whereas immunocompetent animals were able to clear the infection with time, immunosuppressed animals demonstrated virus loads in the liver that appeared to be stable over time (Figure 6c). But, again, we did not observe liver toxicity grossly, microscopically, or in serum levels of alanine transaminase and alkaline phosphatase, markers of hepatic damage (data not shown).
Discussion
The finding that immunosuppression significantly enhanced oncolytic Ad efficacy is very interesting, particularly considering that the tumors at treatment initiation were very large (>1 ml) for rodent cancer models. This result supports the hypothesis that the primary mechanism by which oncolytic Ads suppress tumor growth is by lysis of infected cells as part of the natural viral life cycle. Our data further suggest that the host immune response did not contribute significantly to efficacy, e.g., by eliminating infected cells or by inducing antitumor immunity.
In addition to the observation of improved oncolytic Ad efficacy with immunosuppression, the timing of the efficacy improvement is informative. Initially, tumor suppression was similar in animals receiving oncolytic Ad, regardless of immune status. Only later, in the period of 2–3 weeks, did the difference in efficacy begin to be evident. This timing suggests that as the acquired immune response against Ad developed in immunocompetent animals, virus was cleared from the tumor and tumors resumed growth. Indeed, the data on intratumoral virus levels support this concept, as virus levels were similar among immunocompetent and immunosuppressed animals through 9 days, but by 22 days, intratumoral virus levels dropped significantly in immunocompetent animals. This result, along with the immunohistochemical data, suggests that the cycle of virus replication and infection of additional tumor cells continues in immunosuppressed animals, while, in immunocompetent animals the virus is cleared, preventing infection of remaining tumor cells.
As a whole, these data suggest an inverse relationship between virus level and tumor volume. Multiple mechanisms of Ad-induced tumor suppression may exist in addition to oncolysis, such as a cytotoxic effect of an Ad protein or perhaps an effect on angiogenesis. However, these studies indicate that the effects of oncolytic Ads appear to be dependent on virus replication, either by directly causing lysis of infected tumor cells or by amplifying unknown antitumor virus effects. In addition, our data suggest that antitumor Ad effects appear to be countered to some extent by the immune system. Although we studied the mechanism of tumor suppression with a single tumor type, it seems fairly unlikely that with a different tumor cell line, the virus would have an entirely different mechanism of suppressioni.e., oncolysis versus immune response mediated. We have observed similar efficacy and histopathology in other hamster tumors injected with oncolytic Ad, in which we see primarily necrosis and minimal immune cell infiltration and apoptosis.
Another interesting aspect of the tumor data is the consistency with which tumor growth was controlled in immunosuppressed animals that received oncolytic Ad. A common problem known to basic science and clinical investigators alike is the variability observed in tumor response to therapy. We, and others, typically observe a range of tumor responses and have been interested in understanding the basis for this variability. We also observed considerable differences in intratumoral virus titers in immunocompetent animals at the 22 day time point. It is possible that the variation in efficacy of oncolytic Ads can be partially explained by the character of the immune response and rate of virus clearance in each host. Interestingly, Ad5 and VRX-007 demonstrated similar efficacy in these studies. Although we know that the Ad death protein functions in hamster cells, we do not yet know whether other E3 proteins are able to exert their functions fully in hamster cells in vitro or in vivo. Thus, we cannot yet fully compare the properties of these two vectors in hamsters.
In immunosuppressed hamsters, the level of virus in tumors remains elevated, but does not appear to increase with time. We know that some virus continues to leak out of the tumor into adjacent tissues and the bloodstream and travel to distant sites. Also, it is unclear what fraction of the replicating virus within the tumor is being titered, as each time point is only a snapshot of the number of infectious viral particles that have been produced and have not yet infected new cells. Interestingly, a study of oncolytic Ad replication in human A549 xenografts in nude mice showed very similar results.31 In that study, virus levels in the tumor did not increase significantly with time. In fact, the amount of virus isolated in the human xenograft tumors is nearly the same as the level we isolated from hamster tumors in immunosuppressed animals in our studies. Other studies in nude mice have also showed virus presence in the tumors at late time points.32
Another finding of note was that although the quantitative PCR titration showed the same trends as infectious titration, at later time points in immunocompetent animals, genome levels remained somewhat elevated when infectious virus levels had declined considerably. Because PCR detects a short DNA segment, viral DNA in infected cells that have undergone apoptosis or necrosis may still be detected until this debris is cleared from the tumor. This data suggests that Ad DNA was cleared more slowly than infectious virus and cautions the use of real-time PCR as a direct correlate for infectious virus quantitation in vivo. Also, although the infectious assay may not detect virus that is neutralized by antibody, this is acceptable because such complexes are nonfunctional in the host.
The kinetics of virus clearance from the tumor in immunocompetent animals suggests that the acquired immune response may be primarily responsible for the diminishing efficacy seen at later time points. Although the immune system likely controls virus growth by a coordinated effort from all components, depletion of specific leukocyte subsets may define a crucial cell population. Although the animals in this study did not show signs of opportunistic infections, depletion of a specific leukocyte population may be preferred, if considered in humans.
The data on virus levels in the blood and liver are interesting in many ways. Although the virus replicates in the liver, no adverse effects were observed. The animals appeared well, no toxicity was observed by gross or histological inspection, and serum levels of alanine transaminase and alkaline phosphatase were normal. We feel that virus replication in the liver is rather an asset to the model in that the mechanism of hepatic toxicity can be studied in a permissive immunocompetent animal.
It is also interesting that the levels of Ad genomes in the blood of immunosuppressed hamsters were similar to those reported in immunocompromised individuals with disseminated Ad infection33–35 The degree of immunosuppression was also similar to that observed in patients that develop systemic Ad infections.33–36 These parallels may allow the immunosuppressed hamster to model this clinically important disease in a permissive context.
In summary, we found that immunosuppression allowed sustained Ad replication and oncolysis and led to significantly improved tumor suppression. The remarkable tumor responses seen with oncolytic Ads in immunosuppressed hamsters with large tumors are very promising. Efficacy with Ad vectors in clinical trials thus far has been somewhat limited. This may be in part due to the challenge of large tumor burden, relatively low vector doses, and an immunocompetent individual. Combination therapy with CP and oncolytic Ad may be efficacious in humans as well. Because many chemotherapy drugs are associated with immunosuppression, the combination of oncolytic Ad and such chemotherapy agents may be useful. This may yield oncolytic Ad antitumor effects, chemotherapy antitumor effects, and immunosuppression enhancement of Ad effects. The potential benefit of immunosuppression must be carefully weighed against the risk of systemic Ad infection. Further study of these issues in the hamster model may be valuable in this regard.
Materials and Methods
Cell culture and viruses
The Syrian hamster HaK renal cell line and the human A549 lung carcinoma cell line were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum as previously described.4 The oncolytic Ad vector VRX-007 (ref. 27) is identical to Ad5, except that VRX-007 lacks most of the E3 genes37 and overexpresses the E3-11.6K Ad death protein.28 Viruses were titered by concurrent plaque assays on A549 cells.
Animals
Syrian hamsters (4–5 weeks old) were obtained from Harlan Sprague Dawley (Indianapolis, IN). Studies were approved by the Animal Care Committee of Saint Louis University and were conducted in accordance with institutional and federal regulations.
Immunosuppression
A stock solution of CP (42 mg/ml) was dissolved in water and sterile filtered (Sigma-Aldrich, St Louis, MO). Before use, the stock solution was diluted 1:1 with sterile 1.8% NaCl (2× “normal saline”) to a final CP concentration of 21 mg/ml. The stock and working drug solutions were stored at 4°C in the dark. CP was administered twice per week by intraperitoneal injection for the duration of the study. Before injection, the area was swabbed with 70% ethanol. The initial dose was 140 mg/kg body weight, with 100 mg/kg used thereafter. The dose and schedule was designed based on published studies of CP immunosuppression in mice and hamsters.38–40
Antibacterial prophylaxis
Several precautions were taken for all animals in studies in which CP was used. Animals were housed in sterile microisolator caging and were fed irradiated chow. The antibiotic Baytril was added to sterile drinking water at a final concentration of 100 mg/l. Baytril injectable solution (2.27%) was obtained from Bayer HealthCare (Shawnee Mission, KS). Further, animals in these studies were always handled in the biological safety cabinet, and gloves were sprayed with disinfectant upon entrance into the cabinet and before handling animals.
Antitumor efficacy
HaK tumors were formed by subcutaneous injection of 2 × 107 cells into the hindflank of hamsters. Animals were randomized based on tumor volume before initiation of intratumoral virus injections (mean initial tumor volume = 1,294 µl). Established tumors were injected intratumorally with 1 × 1010 plaque-forming units of VRX-007 or Ad5, or vehicle on days 0, 1, 2, and 4–6. Tumors were monitored by measurement with digital calipers.41
Blood collection and analysis
Blood was collected from anesthetized animals via the jugular vein.41 Hematology and serum chemistry analysis was performed by the Clinical Pathology laboratory in the Department of Comparative Medicine at Saint Louis University. Serum for neutralizing antibody quantitation was obtained at 22 days after initial virus injection and at study termination.
Histopathology and immunohistochemistry
The tumor, liver, kidney, adrenal, spleen, pancreas, heart, lungs, thymus, salivary gland, and mesenteric lymph node of each animal were preserved in 10% neutral-buffered formalin. After fixation, tissues were processed and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin for pathology assessment by an investigator who was blinded to the treatment group. For immunohistochemistry, unstained slides were prepared from formalin-fixed, paraffin-embedded tissues. All immunohistochemistry staining was performed by the histopathology laboratory with an automated system. Slides were incubated with 3% hydrogen peroxide to quench endogenous peroxidase activity, and antigen retrieval was performed with Proteinase K. Primary antibody incubations were performed at a 1:500 dilution. The primary antibody used was mouse anti-Ad hexon antibody (Chemicon, Temecula, CA) or an isotype control mouse IgG1 (Chemicon, Temecula, CA). Secondary antibody incubation was performed with goat anti-mouse IgG (horseradish peroxidase conjugated) from Dako (Envision + system; Carpinteria, CA). Slides were incubated with diaminobenzidine substrate and counterstained with hematoxylin.
Assessment of virus quantities in tissues
Animals were killed and organs were harvested in order of probable increasing virus titer, using new sterile instruments for each animal. Blood was collected in anticoagulant tubes as well as in 6-ml snap-cap tubes and frozen in a dry ice-ethanol bath; other organs were also collected in sterile tubes and frozen. Net weight of all organs was recorded. Before homogenization, tumors were minced due to their firm nature. Tumor and liver samples were divided into multiple tubes, as necessary, for homogenization. Homogenization of organs in phosphate-buffered saline (PBS) was conducted with a single tungsten carbide bead with the TissueLyser (Qiagen, Valencia, CA). For real-time PCR quantitation, DNA was isolated from each homogenate with the Magtration system (PSS BioInstruments, Livermore, CA). After DNA quantification with a PicoGreen assay (Invitrogen, Carlsbad, CA), real-time PCR was performed with the ABI 7500 system (Applied Biosystems, Foster City, CA), with 1 µg DNA per reaction when possible. For infectious titer determination, homogenates were frozen and thawed three times, sonicated, centrifuged, and titered by tissue culture infectious dose 50 assays on A549 cells. Each sample was titered in a single 96-well plate. At 14 days after infection, 96-well plates were scored (±) for cytopathic effect and titers were calculated according to the Reed–Muench method.
Neutralizing antibody titration
One day before the assay, A549 cells were plated at 8.33 × 103 cells/well in a volume of 100 µl in 96-well plates. Serum samples were incubated at 56°C for 30 minutes to inactivate complement. Two serum samples were assayed per plate. Serum samples (in four replicate wells) were diluted twofold across a round-bottom 96-well plate, in media containing 20% fetal bovine serum to normalize the total serum concentration across the plate.42,43 One column contained no serum sample to observe the effect of virus only. Dilutions of sera were incubated with 100 plaque-forming units/well of VRX-007 for 1 hour at 37°C. After 1 hour of incubation, the serum-virus mixtures (100 µl total volume) were transferred to the 96-well plate containing A549 cells. After infection for 1 hour at 37°C, the media was removed and replaced with fresh Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum. Wells were individually scored (±) for cytopathic effect at 6 days after infection. Neutralizing titers were determined by the highest dilution of serum that resulted in at least 50% inhibition of cytopathic effect (less than or equal to two of four wells positive for cytopathic effect).
Statistical analysis
We compared the data from multiple groups by one-way analysis of variance, with a Tukey post hoc test to evaluate differences between groups. In the event that a significant difference was detected with the test of homogeneity of variances (i.e., analysis of variance not valid), a Kruskal–Wallis test was followed by pairwise comparison of groups with the Mann–Whitney U-test. Significant differences were defined as P < 0.05.
Acknowledgments
We thank Jennifer Meyer for her assistance in processing tissues and preparing slides for analysis and Elsa Taricone for excellent animal care. We also thank Emma Thomas for her assistance with real-time PCR. This research was supported by grants CA118022 and CA108335 from the National Institutes of Health, and also by a grant from VirRx, Inc., to Saint Louis University. W.S.M.W. and K.T. own stock in VirRx, Inc. Saint Louis University owns patents on the vector VRX-007. VirRx holds a worldwide exclusive license to these patents.
References
- 1.Freytag SO, Stricker H, Movsas B, Kim JH. Prostate cancer gene therapy clinical trials. Mol Ther. 2007;15:1042–1052. doi: 10.1038/sj.mt.6300162. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein DL, Wold WSM. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 2004;11:819–829. doi: 10.1038/sj.cgt.7700765. [DOI] [PubMed] [Google Scholar]
- 3.Lin E, Nemunaitis J. Oncolytic viral therapies. Cancer Gene Ther. 2004;11:643–664. doi: 10.1038/sj.cgt.7700733. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 5.Toth K, Djeha H, Ying BL, Tollefson AE, Kuppuswamy M, Doronin K, et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated Wnt signaling. Cancer Res. 2004;64:3638–3644. doi: 10.1158/0008-5472.CAN-03-3882. [DOI] [PubMed] [Google Scholar]
- 6.Relph KL, Harrington KJ, Pandha H. Adenoviral strategies for the gene therapy of cancer. Semin Oncol. 2005;32:573–582. doi: 10.1053/j.seminoncol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 8.Thorne SH, Hermiston T, Kirn D. Oncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effects. Semin Oncol. 2005;32:537–548. doi: 10.1053/j.seminoncol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, McCormick F, Lang FF, Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert Rev Anticancer Ther. 2006;6:697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- 10.Assouline S, Sylvestre MP, Carriere P, Shustik C, Laneuville P. Comparison of peripheral blood progenitor cell yield from standard chemotherapy used in the treatment of lymphoid malignancies and high-dose cyclophosphamide: a retrospective review of 141 patients. Transfusion (Paris) 2006;46:174–179. doi: 10.1111/j.1537-2995.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 11.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Ferry C, Socie G. Busulfan-cyclophosphamide versus total body irradiation-cyclophosphamide as preparative regimen before allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia: what have we learned? Exp Hematol. 2003;31:1182–1186. doi: 10.1016/j.exphem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM. Reduced-intensity transplantation for lymphoma. Curr Treat Options Oncol. 2006;7:295–305. doi: 10.1007/s11864-006-0039-0. [DOI] [PubMed] [Google Scholar]
- 14.Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peruzzotti G, et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006;17:961–967. doi: 10.1097/01.cad.0000224454.46824.fc. [DOI] [PubMed] [Google Scholar]
- 15.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 16.Tada K, Ito Y, Takahashi S, Iijima K, Miyagi Y, Nishimura S, et al. Tolerability and safety of classic cyclophosphamide, methotrexate, and Fluorouracil treatment in Japanese patients with early breast cancer. Breast Cancer. 2006;13:279–283. doi: 10.2325/jbcs.13.279. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44:1135–1164. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 18.Parsons PG, Lean J, Kable EP, Favier D, Khoo SK, Hurst T, et al. Relationships between resistance to cross-linking agents and glutathione metabolism, aldehyde dehydrogenase isozymes and adenovirus replication in human tumour cell lines. Biochem Pharmacol. 1990;40:2641–2649. doi: 10.1016/0006-2952(90)90582-6. [DOI] [PubMed] [Google Scholar]
- 19.Bouvet M, Fang B, Ekmekcioglu S, Ji L, Bucana CD, Hamada K, et al. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low-dose etoposide. Gene Ther. 1998;5:189–195. doi: 10.1038/sj.gt.3300564. [DOI] [PubMed] [Google Scholar]
- 20.Jooss K, Yang Y, Wilson JM. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 21.Smith TA, White BD, Gardner JM, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 22.Di Paolo NC, Tuve S, Ni S, Hellstrom KE, Hellstrom I, Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 26.Smakman N, van der Bilt JD, van den Wollenberg DJ, Hoeben RC, Borel Rinkes I, Kranenburg O. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13:815–818. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]
- 27.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WSM. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 28.Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WSM. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WSM. The E3-11.6kDa Adenovirus Death Protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 30.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005;39:105–112. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum Gene Ther. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 32.Harrison D, Sauthoff H, Heitner S, Jagirdar J, Rom WN, Hay JG. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved—deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum Gene Ther. 2001;12:1323–1332. doi: 10.1089/104303401750270977. [DOI] [PubMed] [Google Scholar]
- 33.Heemskerk B, Lankester AC, van Vreeswijk T, Beersma MF, Claas EC, Veltrop-Duits LA, et al. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J Infect Dis. 2005;191:520–530. doi: 10.1086/427513. [DOI] [PubMed] [Google Scholar]
- 34.Kampmann B, Cubitt D, Walls T, Naik P, Depala M, Samarasinghe S, et al. Improved outcome for children with disseminated adenoviral infection following allogeneic stem cell transplantation. Br J Haematol. 2005;130:595–603. doi: 10.1111/j.1365-2141.2005.05649.x. [DOI] [PubMed] [Google Scholar]
- 35.Watzinger F, Suda M, Preuner S, Baumgartinger R, Ebner K, Baskova L, et al. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J Clin Microbiol. 2004;42:5189–5198. doi: 10.1128/JCM.42.11.5189-5198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Tol MJ, Claas EC, Heemskerk B, Veltrop-Duits LA, de Brouwer CS, van Vreeswijk T, et al. Adenovirus infection in children after allogeneic stem cell transplantation: diagnosis, treatment and immunity. Bone Marrow Transplant. 2005;35(suppl. 1):S73–S76. doi: 10.1038/sj.bmt.1704852. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WSM. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- 38.Blandford G, Charlton D. Studies of pulmonary and renal immunopathology after nonlethal primary sendai viral infection in normal and cyclophosphamide-treated hamsters. Am Rev Respir Dis. 1977;115:305–314. doi: 10.1164/arrd.1977.115.2.305. [DOI] [PubMed] [Google Scholar]
- 39.Kercher L, Mitchell BM. Immune transfer protects severely immunosuppressed mice from murine cytomegalovirus retinitis and reduces the viral load in ocular tissue. J Infect Dis. 2000;182:652–661. doi: 10.1086/315781. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell BM, Stevens JG. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J Immunol. 1996;156:246–255. [PubMed] [Google Scholar]
- 41.Thomas MA, Spencer JF, Wold WSM. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. In: Tollefson AE, Wold WSM, editors. Adenovirus Methods and Protocols. 2nd edn. Humana: St. Louis; 2006. pp. 169–183. [DOI] [PubMed] [Google Scholar]
- 42.Aste-Amezaga M, Bett AJ, Wang F, Casimiro DR, Antonello JM, Patel DK, et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther. 2004;15:293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 43.Zhi Y, Figueredo J, Kobinger GP, Hagan H, Calcedo R, Miller JR, et al. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum Gene Ther. 2006;17:500–506. doi: 10.1089/hum.2006.17.500. [DOI] [PubMed] [Google Scholar]