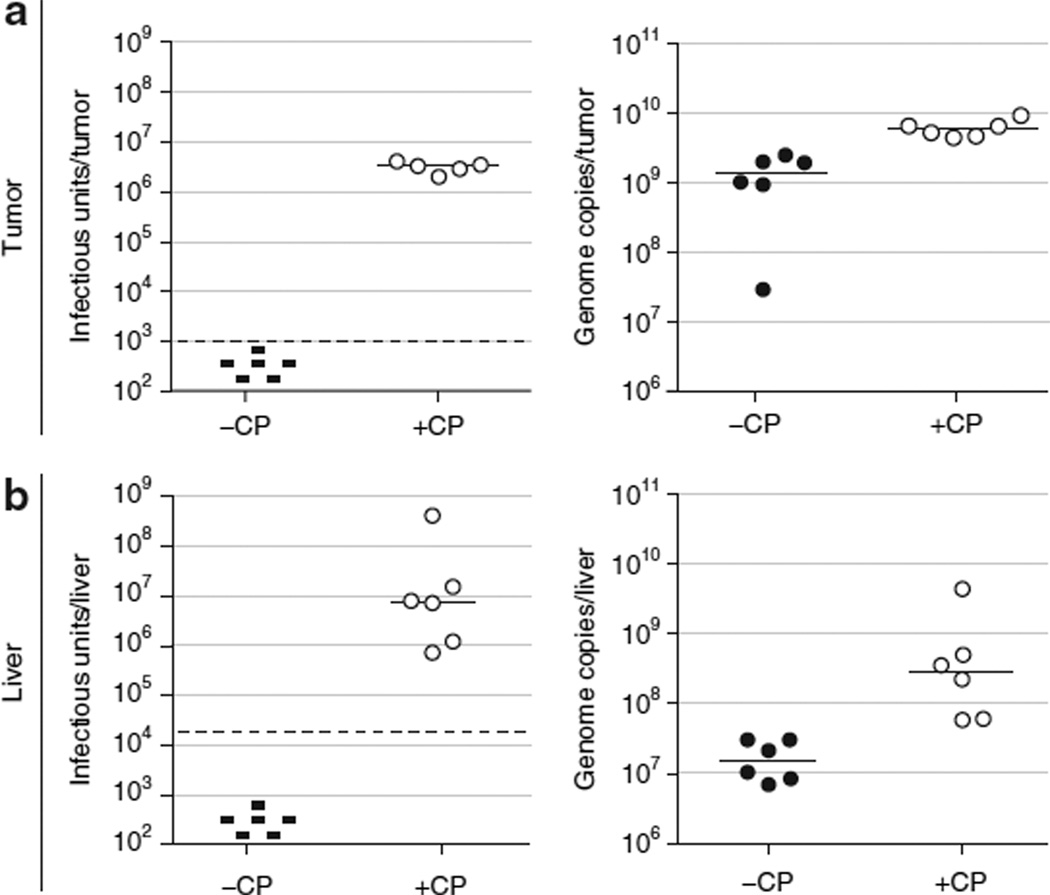
Figure 5. Immunosuppression allowed virus levels to remain elevated for the duration of the antitumor efficacy study, while virus was cleared in immunocompetent animals.
The tumor and a portion of the liver from 6 of 12 animals in the “VRX-007−” and “VRX-007 + CP” groups in the antitumor efficacy study (Figure 2) were obtained at study termination for virus quantitation. Tumor and liver samples were harvested and processed for virus titration by a tissue culture infectious dose 50 (TCID50) infectious assay as well as a quantitative real-time PCR assay for adenovirus (Ad) genomes. The titers obtained from (a) tumor and (b) liver specimens are shown. Left panels, TCID50 infectious titration. Right panels, quantitative real-time PCR titration. Each point represents the titer from one animal and the bars indicate the median for each group. The threshold for the TCID50 assay is shown by the dashed horizontal line. Samples in which no infectious virus was detected are indicated by “−.” CP, cyclophosphamide.