Abstract
A variety of experimental data on synthetase-tRNA interactions are examined. Although these data previously had no direct explanation when viewed only in terms of the tRNA cloverleaf diagram, they can be rationalized according to a simple proposal that takes account of the three dimensional structure of tRNA. It is proposed that a major part of the binding site for most or all synthetases is along and around the diagonal side of the tRNA structure, which contains the acceptor stem, dihydrouridine stem, and anticodon. This side of the tRNA molecule contains structural features likely to be common for all tRNAs. Depending on the system, an enzyme may span a small part or all of the region of this side of the molecule. Interactions with other parts of the structure may also occur in a manner that varies from complex to complex. These interactions may be determined, in part, by the angle at which the diagonal side of the flat tRNA molecule is inserted onto the surface of the synthetase.
Full text
PDF

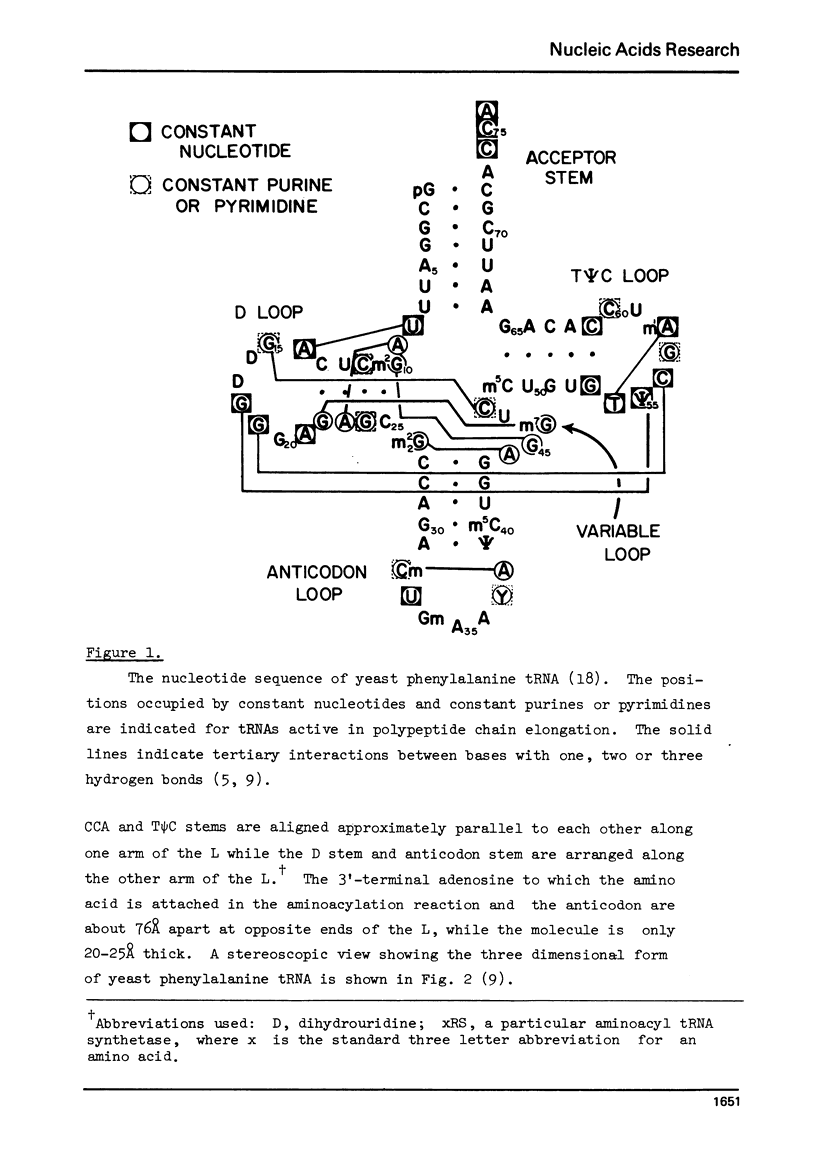
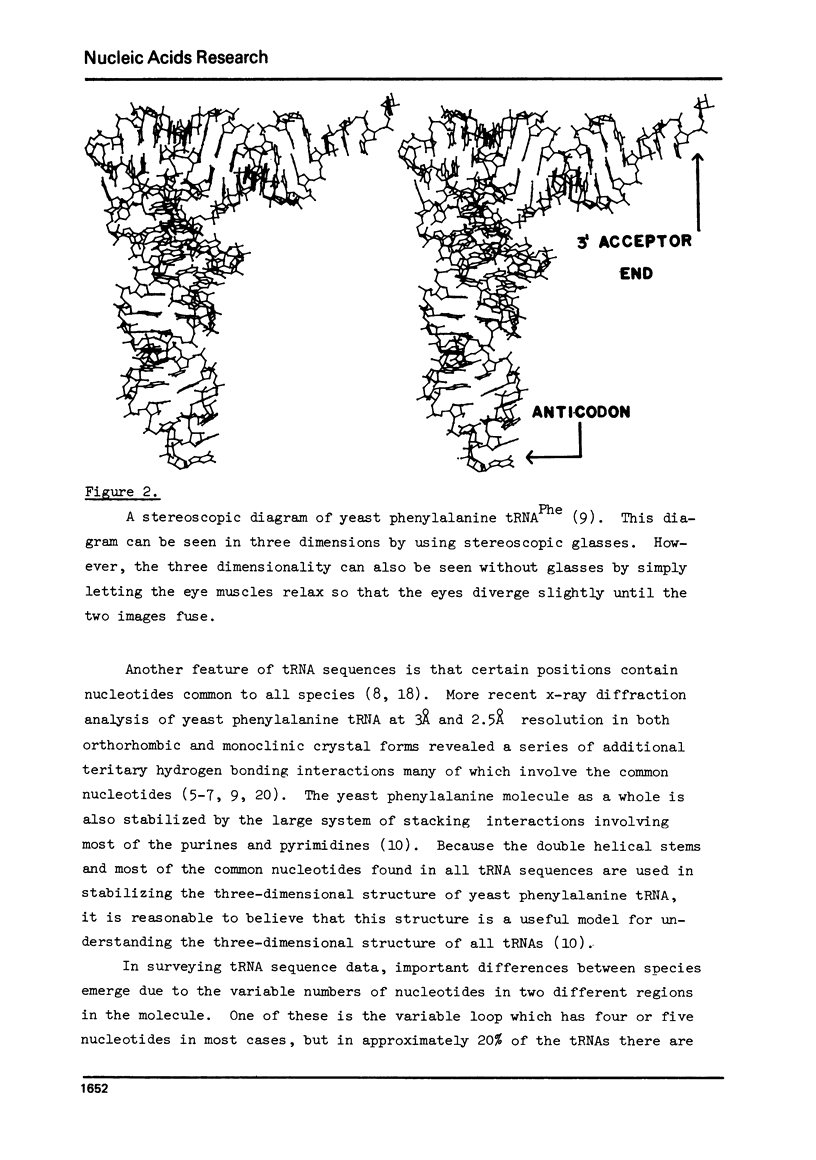

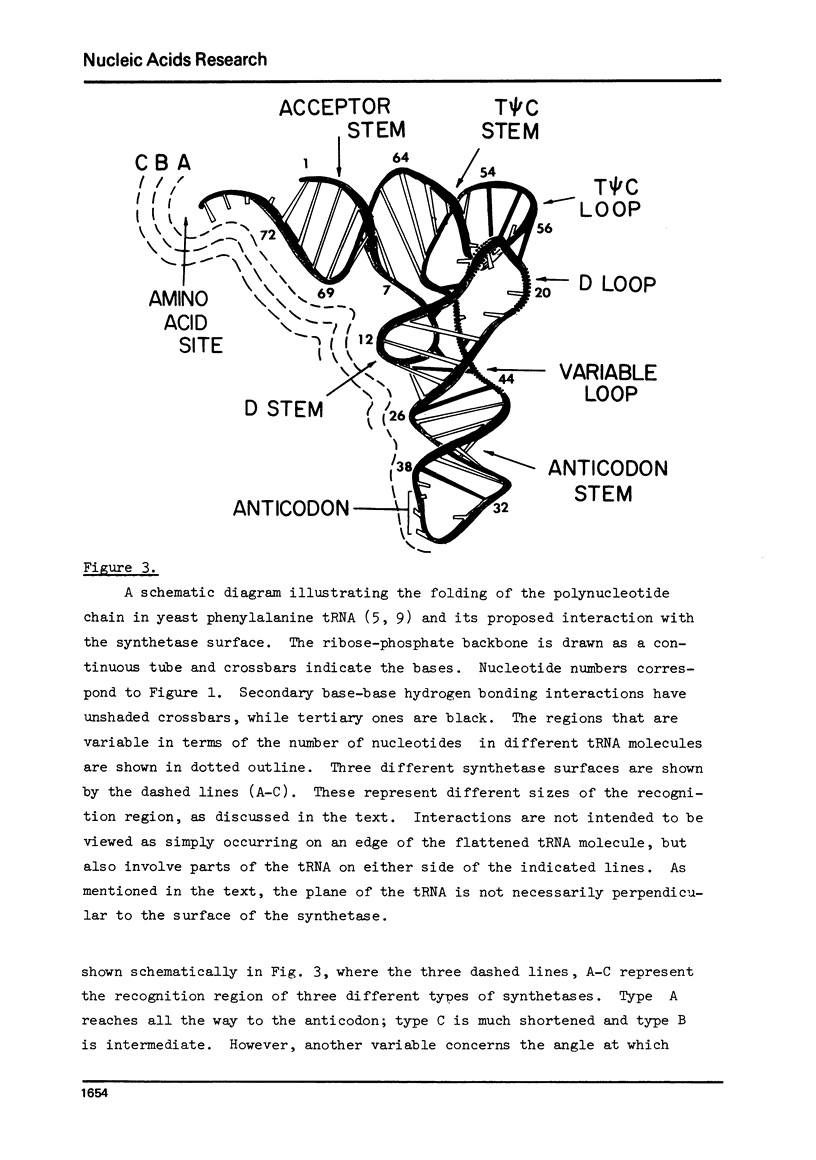











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budzik G. P., Lam S. S., Schoemaker H. J., Schimmel P. R. Two photo-cross-linked complexes of isoleucine specific transfer ribonucleic acid with aminoacyl transfer ribonucleic acid synthetases. J Biol Chem. 1975 Jun 25;250(12):4433–4439. [PubMed] [Google Scholar]
- CHAPEVILLE F., LIPMANN F., VON EHRENSTEIN G., WEISBLUM B., RAY W. J., Jr, BENZER S. On the role of soluble ribonucleic acid in coding for amino acids. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1086–1092. doi: 10.1073/pnas.48.6.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré D. S., Thomas G., Favre A. Conformation and functioning of tRNAs: cross-linked tRNAs as substrate for tRNA nucleotidyl-transferase and aminoacyl synthetases. Biochimie. 1974;56(8):1089–1101. doi: 10.1016/s0300-9084(74)80097-0. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudock B., DiPeri C., Scileppi K., Reszelbach R. The yeast phenylalanyl-transfer RNA synthetase recognition site: the region adjacent to the dihydrouridine loop. Proc Natl Acad Sci U S A. 1971 Mar;68(3):681–684. doi: 10.1073/pnas.68.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J. P., Giegé R., Bonnet J., Kern D., Befort N., Bollack C., Fasiolo F., Gangloff J., Dirheimer G. Factors determining the specificity of the tRNA aminoacylation reaction. Non-absolute specificity of tRNA-aminoacyl-tRNA synthetase recognition and particular importance of the maximal velocity. Biochimie. 1973 May;55(5):547–557. doi: 10.1016/s0300-9084(73)80415-8. [DOI] [PubMed] [Google Scholar]
- Giege R., Kern D., Ebel J. P., Taglang R. Incorrect heterologous aminoacylation of various yeast tRNAS catalysed by E. coli valyl-tRNA synthetase. FEBS Lett. 1971 Jul 1;15(4):281–285. doi: 10.1016/0014-5793(71)80638-5. [DOI] [PubMed] [Google Scholar]
- Giegé R., Kern D., Ebel J. P., Grosjean H., de Henau S., Chantrenne H. Incorrect aminoacylations involving tRNAs or valyl-tRNA synthetase from Bacillus stearothermophilus. Eur J Biochem. 1974 Jun 15;45(2):351–362. doi: 10.1111/j.1432-1033.1974.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Giegé R., Kern D., Ebel J. P. Incorrect aminoacylations catalysed by E. coli valyl-tRNA synthetase. Biochimie. 1972;54(10):1245–1255. doi: 10.1016/s0300-9084(72)80065-8. [DOI] [PubMed] [Google Scholar]
- HOLLEY R. W., APGAR J., EVERETT G. A., MADISON J. T., MARQUISEE M., MERRILL S. H., PENSWICK J. R., ZAMIR A. STRUCTURE OF A RIBONUCLEIC ACID. Science. 1965 Mar 19;147(3664):1462–1465. doi: 10.1126/science.147.3664.1462. [DOI] [PubMed] [Google Scholar]
- Hooper M. L., Russell R. L., Smith J. D. Mischarging in mutant tyrosine transfer RNAs. FEBS Lett. 1972 Apr 15;22(1):149–155. doi: 10.1016/0014-5793(72)80241-2. [DOI] [PubMed] [Google Scholar]
- Irwin M. J., Nyborg J., Reid B. R., Blow D. M. The crystal structure of tyrosyl-transfer RNA synthetase at 2-7 A resolution. J Mol Biol. 1976 Aug 25;105(4):577–586. doi: 10.1016/0022-2836(76)90236-9. [DOI] [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA's with different codon responses. Biochim Biophys Acta. 1971 Jan 28;228(2):471–481. doi: 10.1016/0005-2787(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Ebel J. P. Incorrect aminoacylatins catalysed by the phenylalanyl-and valyl-tRNA synthetases from yeast. Eur J Biochem. 1972 Nov 21;31(1):148–155. doi: 10.1111/j.1432-1033.1972.tb02513.x. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Quigley G. J., Suddath F. L., McPherson A., Sneden D., Kim J. J., Weinzierl J., Rich A. Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain. Science. 1973 Jan 19;179(4070):285–288. doi: 10.1126/science.179.4070.285. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Symmetry recognition hypothesis model for tRNA binding to aminoacyl-tRNA synthetase. Nature. 1975 Aug 21;256(5519):679–681. doi: 10.1038/256679a0. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. S., Schimmel P. R. Equilibrium measurements of cognate and noncognate interactions between aminoacyl transfer RNA synthetases and transfer RNA. Biochemistry. 1975 Jun 17;14(12):2775–2780. doi: 10.1021/bi00683a034. [DOI] [PubMed] [Google Scholar]
- Litvak S., Tarragó A., Tarragó-Litvak L., Allende J. E. Elongation factor-viral genome interaction dependent on the aminoacylation of TYMV and TMV RNAs. Nat New Biol. 1973 Jan 17;241(107):88–90. doi: 10.1038/newbio241088a0. [DOI] [PubMed] [Google Scholar]
- Makman M. H., Cantoni G. L. Studies concerning the interaction of serine soluble ribonucleic acid with seryl soluble ribonucleic acid synthetase from baker's yeast. Biochemistry. 1966 Jul;5(7):2246–2254. doi: 10.1021/bi00871a013. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Bayev A. A. Investigation of recognition sites in valine tRNA I (Baker's yeast) by dissected molecule method. Methods Enzymol. 1974;29:643–661. doi: 10.1016/0076-6879(74)29056-6. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Lastity D., Levina E. S., Bayev A. A. Localization of two recognition sites in yeast valine tRNA I. Nat New Biol. 1971 Jan 6;229(1):21–22. doi: 10.1038/newbio229021a0. [DOI] [PubMed] [Google Scholar]
- Oberg B., Philipson L. Binding of histidine to tobacco mosaic virus RNA. Biochem Biophys Res Commun. 1972 Aug 21;48(4):927–932. doi: 10.1016/0006-291x(72)90697-3. [DOI] [PubMed] [Google Scholar]
- Pinck M., Chan S. K., Genevaux M., Hirth L., Duranton H. Valine specific tRNA-like structure in RNAs of two viruses of Turnip Yellow Mosaic Virus group. Biochimie. 1972;54(8):1093–1094. doi: 10.1016/s0300-9084(72)80062-2. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Seeman N. C., Suddath F. L., Rich A., Sussman J. L., Kim S. H. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4866–4870. doi: 10.1073/pnas.72.12.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Rich A. The three-dimensional structure of yeast phenylalanine transfer ribonucleic acid and its interaction with aminoacyl synthetases. Biochem Soc Trans. 1975;3(5):641–645. doi: 10.1042/bst0030641. [DOI] [PubMed] [Google Scholar]
- Rich A. Transfer RNA and protein synthesis. Biochimie. 1974;56(11-12):1441–1449. doi: 10.1016/s0300-9084(75)80265-3. [DOI] [PubMed] [Google Scholar]
- Rigler R., Cronvall E., Hirsch R., Pachmann U., Zachau H. G. Interactions of seryl-tRNA synthetase with serine and phenylalanine specific tRNA. FEBS Lett. 1970 Dec 18;11(5):320–323. doi: 10.1016/0014-5793(70)80558-0. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Roe B., Sirover M., Dudock B. Kinetics of homologous and heterologous aminoacylation with yeast phenylalanyl transfer ribonucleic acid synthetase. Biochemistry. 1973 Oct 9;12(21):4146–4154. doi: 10.1021/bi00745a018. [DOI] [PubMed] [Google Scholar]
- Salomon R., Littauer U. Z. Enzymatic acylation of histidine to mengovirus RNA. Nature. 1974 May 3;249(452):32–34. doi: 10.1038/249032a0. [DOI] [PubMed] [Google Scholar]
- Samuelson G., Keller E. B. Excision of nucleotides from the dihydrouridine loop of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1972 Jan 4;11(1):30–35. doi: 10.1021/bi00751a006. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Buchardt B., Reid B. R. Effect of cleaving the dihydrouridine loop and the ribothymidine loop on the amino acid acceptor activity of yeast phenylalanine transfer ribonucleic acid. J Biol Chem. 1970 Nov 10;245(21):5743–5750. [PubMed] [Google Scholar]
- Schoemaker H. J., Budzik G. P., Giegé R., Schimmel P. R. Three photo-cross-linked complexes of yeast phenylalanine specific transfer ribonucleic acid with aminoacyl transfer ribonucleic acid synthetases. J Biol Chem. 1975 Jun 25;250(12):4440–4444. [PubMed] [Google Scholar]
- Schoemaker H. J., Schimmel P. R. Isotope labeling of free and aminoacyl transfer RNA synthetase-bound transfer RNA. J Biol Chem. 1976 Nov 10;251(21):6823–6830. [PubMed] [Google Scholar]
- Schoemaker H. J., Schimmel P. R. Photo-induced joining of a transfer RNA with its cognate aminoacyl-transfer RNA synthetase. J Mol Biol. 1974 Apr 25;84(4):503–513. doi: 10.1016/0022-2836(74)90112-0. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Goddard J. P. Loss of methionine acceptor activity resulting from a base change in the anticodon of Escherichia coli formylmethionine transfer ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1341–1345. [PubMed] [Google Scholar]
- Shimura Y., Aono H., Ozeki H., Sarabhai A., Lamfrom H., Abelson J. Mutant tyrosine tRNA of altered amino acid specificity. FEBS Lett. 1972 Apr 15;22(1):144–148. doi: 10.1016/0014-5793(72)80240-0. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Celis J. E. Mutant tyrosine transfer RNA that can be charged with glutamine. Nat New Biol. 1973 May 16;243(124):66–71. [PubMed] [Google Scholar]
- Squires C., Carbon J. Normal and mutant glycine transfer RNAs. Nat New Biol. 1971 Oct 27;233(43):274–277. doi: 10.1038/newbio233274a0. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Rogg H., Baguley B. C., Ginsberg T., Wehrli W. Structure of a mammalian serine tRNA. Nature. 1968 Sep 28;219(5161):1363–1365. doi: 10.1038/2191363a0. [DOI] [PubMed] [Google Scholar]
- Thiebe R., Harbers K., Zachau H. G. Aminoacylation of fragment combinations from yeast tRNA phe . Eur J Biochem. 1972 Mar 15;26(1):144–152. doi: 10.1111/j.1432-1033.1972.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. A specific chemical chain scission of tRNA at 7-methylguanosine. FEBS Lett. 1970 Dec;11(3):160–164. doi: 10.1016/0014-5793(70)80518-x. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Folk W. R., Berg P., Soll L. A single mutational modification of a tryptophan-specific transfer RNA permits aminoacylation by glutamine and translation of the codon UAG. J Mol Biol. 1974 Jun 25;86(2):245–260. doi: 10.1016/0022-2836(74)90016-3. [DOI] [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by aminoacyl tRNA synthetases. J Mol Biol. 1967 Sep 28;28(3):479–490. doi: 10.1016/s0022-2836(67)80098-6. [DOI] [PubMed] [Google Scholar]
- Yarus M. Binding of isoleucyl transfer ribonucleic acid by isoleucyl transfer ribonucleic acid synthetase: solvents, the strength of interaction, and a proposed source of specificity. Biochemistry. 1972 May 23;11(11):2050–2060. doi: 10.1021/bi00761a009. [DOI] [PubMed] [Google Scholar]
- Yarus M. Solvent and specificity. Binding and isoleucylation of phenylalanine transfer ribonucleic acid (Escherichia coli) by isoleucyl transfer ribonucleic acid synthetase from Escherichia coli. Biochemistry. 1972 Jun 6;11(12):2352–2361. doi: 10.1021/bi00762a022. [DOI] [PubMed] [Google Scholar]
- Yot P., Pinck M., Haenni A. L., Duranton H. M., Chapeville F. Valine-specific tRNA-like structure in turnip yellow mosaic virus RNA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1345–1352. doi: 10.1073/pnas.67.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]


