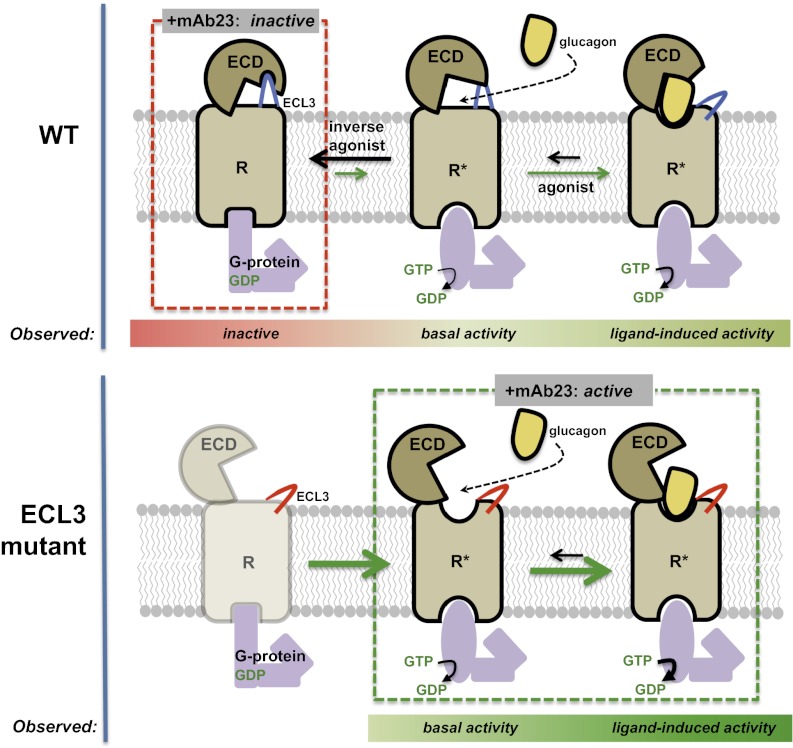
Fig. 6.
In the absence of agonist, GCGR is predominantly in an inactivated state. Basal activity indicates that the receptor is capable of adopting an active conformation, enabling signaling through heterotrimeric G protein nucleotide exchange. Agonist binding stabilizes an active conformation to enable G protein coupling. The ECL3 chimeric receptor is uncoupled from the ECD and more readily adopts an active conformation, even in the absence of agonist. Higher basal and ligand-induced activities are observed in the ECL3 chimera. An inactive conformation of the WT receptor is stabilized by mAb23, an effect lost on the ECL3 chimera.