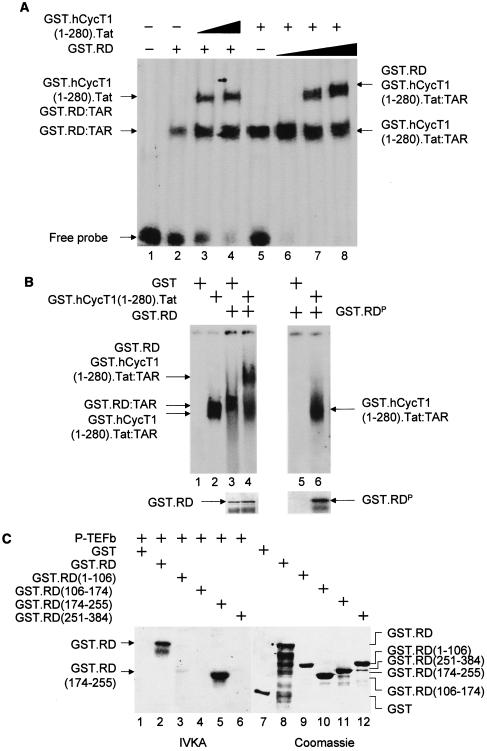
FIG. 2.
Unphosphorylated RD and the complex between Tat and CycT1 (P-TEFb) bind TAR. (A) RD and the complex between CycT1 and Tat bind TAR simultaneously. In lanes 1 to 4, increasing amounts (0.1 and 0.2 μg for lanes 3 and 4, respectively) of the purified GST.hCycT.Tat chimera (hCycT, human CycT) (7) was added to the reaction mixture containing the 32P-labeled HIVTAR and the GST.RD fusion protein. Purified GST protein (0.2 μg) was used as the negative control (lane 2). In lanes 5 to 8, increasing amounts (0.2, 0.5, and 1.0 μg for lanes 2, 3, and 4, respectively) of the purified GST.RD chimera was added to the reaction mixture containing the 32P-labeled HIVTAR and the GST.hCycT.Tat fusion protein (0.1 μg). Purified GST protein (0.2 μg) was used as the negative control (lane 5). The composition of RNA-protein complexes is given next to the arrows pointing to singly shifted and supershifted bands. This EMSA was visualized by the phosphorimager. (B) Phosphorylated RD does not bind TAR in vitro. GST and the GST.RD chimera were phosphorylated by P-TEFb using cold ATP in vitro. As described above, 0.5 μg of unphosphorylated (lanes 1 to 4) and phosphorylated GST.RD (GST.RDP) chimeras (lanes 5 and 6) were used in the absence or presence (+) of the GST.hCycT.Tat fusion protein (0.1 μg) for the EMSA. Phosphorylation was monitored by a parallel experiment using [γ-32P]ATP (lower right panel). After SDS-PAGE, gels were stained with Coomassie brilliant blue to reveal the levels of input proteins (lower left panel). (C) Sequences N terminal to the RRM in RD are phosphorylated by P-TEFb. (Left) RD is phosphorylated by P-TEFb. GST, GST.RD chimera, and its mutant truncated counterparts [GST.RD(1-106), GST.RD(106-174), GST.RD(174-255), and GST.RD(251-384)] were incubated with P-TEFb and [γ-32P]ATP. Proteins were separated by SDS-PAGE, followed by autoradiography. (Right) The input levels of proteins were verified as described for panel B.