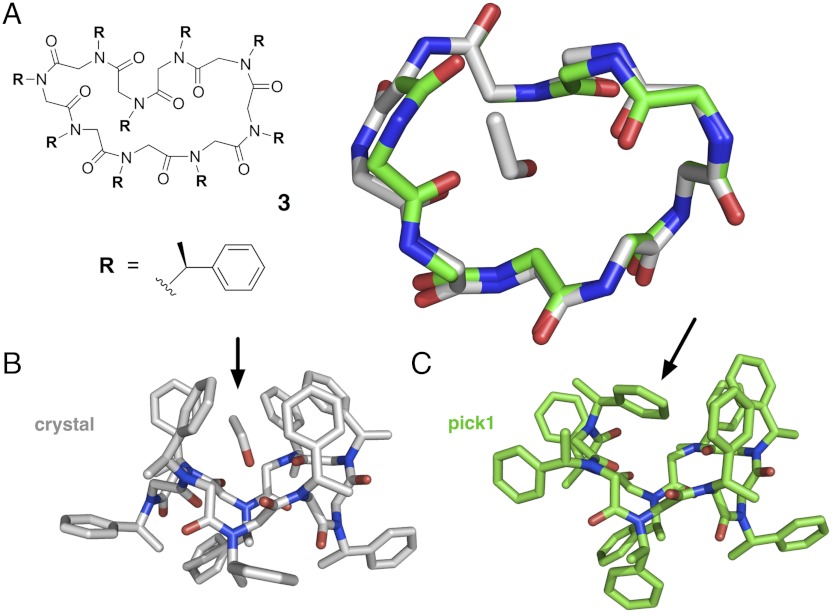
Fig. 4.
Experimental and predicted structures of cyclic peptoid nonamer 3, composed of (S)-N-(1-phenylethyl) glycine units. The amide bonds in the crystal structure were found to exhibit a cis/transpattern of cccctccct, with several significant deviations from amide planarity (see Table 1). (A) Shown superimposed are the backbone conformation of the compound 3 crystal structure (gray) and the top-ranked blind prediction (green), with a backbone-rmsd of approximately 1.0 Å. (B) The crystal structure shows the side chains oriented toward alternating faces of the macrocycle, with five side chain groups forming a “molecular basket” encompassing a single bound ethanol (arrow). (C) The predicted structure, which was modeled in the absence of ethanol, shows instead a peptoid side chain “filling” this basket (arrow), resulting in the rotation of two backbone ψ angles, compared to the crystal structure backbone.