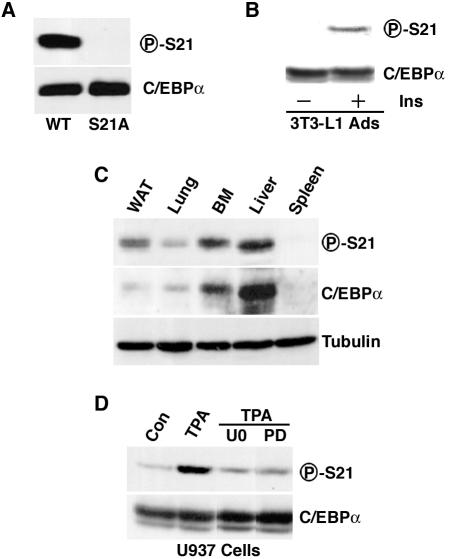
FIG. 3.
Phosphorylation of serine 21 in vivo. (A) CV-1 cells were transfected with an expression vector carrying the gene for either wild-type (WT) or S21A mutant C/EBPα. Lysates were separated by SDS-PAGE and subjected to immunoblot analysis with a phosphospecific antibody designed against serine 21 of C/EBPα (P-S21; top) or total C/EBPα (bottom). (B) After serum deprivation, 3T3-L1 adipocytes (Ads) were not treated (−) or treated (+) with insulin for 10 min. Lysates were separated by SDS-PAGE and subjected to immunoblot analysis for total C/EBPα (bottom) or C/EBPα phosphorylated on serine 21 (top) (C) Lysates from white adipose tissue (WAT), lung, bone marrow (BM), liver, and spleen were separated by SDS-PAGE and subjected to immunoblot analysis with antibody to phosphorylated serine 21 (P-S21) or C/EBPα. (D) U937 cells were washed twice in RPMI medium without serum and suspended in RPMI medium (no serum) at 106/ml. After 2 h at 37°C, cells were treated with 10 μM U0126 (U0) or 50 μM PD98059 (PD) for an additional 2.5 h. TPA was added to a final concentration of 1 μM, and cells were incubated for an additional 30 min. Immunoblot analysis with antisera specific for phosphorylated serine 21 or for C/EBPα was then performed. For all experiments, similar results were obtained in three independent experiments.