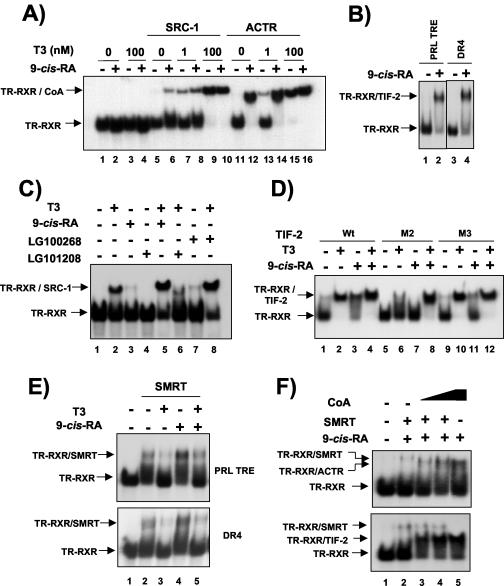
FIG. 7.
RXR agonists cause coactivator recruitment by the RXR/TR heterodimer. (A) Gel retardation assays with the PRL TRE oligonucleotide and in vitro-translated TR and RXR. Assays were performed in the presence of the receptor-interacting domains of the p160 coactivators SRC-1 and ACTR fused to GST. In lanes without coactivators (lanes 1 to 4), the same amount of GST alone was added. As indicated, 9-cis-RA (1 μM) and T3 (1 and 100 nM) were present in the binding assays. Arrows, mobilities of the heterodimer and the complexes containing the heterodimer and the coactivator (CoA). (B) Gel retardation assays were performed with oligonucleotides conforming to either the PRL TRE or a consensus DR4. The binding of the p160 coactivator TIF-2 to RXR/TR was analyzed in the presence and absence of 1 μM 9-cis-RA. (C) Similar assays were performed with SRC-1 in the presence of 100 nM T3 or 1 μM 9-cis-RA, the rexinoid LG100268, or the RXR-selective antagonist LG101208. (D) Association of the RXR/TR heterodimer with wild-type His-tagged TIF-2 or with TIF-2 with mutations in the second (M2) or third (M3) LXXLL motif (450 ng). Assays were performed with the PRL TRE and in the presence of 20 nM T3 and 1 μM 9-cis-RA as indicated. (E) Gel retardation assays with the receptor-interacting domain of the corepressor SMRT fused to GST, the receptor heterodimer, and either the PRL TRE (top) or DR4 (bottom). Assays were performed with 9-cis-RA (1 μM) and/or T3 (20 nM). (F) PRL TRE was incubated with the corepressor in the presence of increasing amounts of GST-ACTR (200 to 600 ng) (top) or His-tagged TIF-2 (300 to 900 ng) (bottom). When indicated, 1 μM 9-cis-RA was present in the assays.