Abstract
Mice lacking CLOCK protein have a relatively subtle circadian phenotype, including a slightly shorter period in constant darkness, differences in phase resetting after 4-hr light pulses in the early and late night, and a variably advanced phase angle of entrainment in a light-dark (LD) cycle (DeBruyne et al., Neuron 50:465–477, 2006). The present series of experiments was conducted to more fully characterize the circadian phenotype of Clock−/− mice under various lighting conditions. A phase-response curve (PRC) to 4-hour light pulses in free-running mice was conducted; the results confirm that Clock−/− mice exhibit very large phase advances after 4 hrs light pulses in the late subjective night, but have relatively normal responses to light at other phases. The abnormal shape of the PRC to light may explain the tendency of CLOCK-deficient mice to begin activity before lights-out when housed in a 12 hrs light: 12 hrs dark lighting schedule. To assess this relationship further, Clock−/− and wild-type control mice were entrained to skeleton lighting cycles (1L:23D, and 1L:10D:1L:12D). Comparing entrainment under the two types of skeleton photoperiods revealed that exposure to 1 hr light in the morning leads to a phase advance of activity onset (expressed the following afternoon) in Clock−/− mice, but not in the controls. Constant light typically causes an intensity-dependent increase in circadian period in mice, but this did not occur in CLOCK-deficient mice. The failure of Clock−/− mice to respond to the period-lengthening effect of constant light likely results from the increased functional impact of light falling in the phase advance zone of the PRC. Collectively, these experiments reveal that alterations in the response of CLOCK-deficient mice to light in several paradigms are likely due to an imbalance in the shape of the PRC to light.
Keywords: Clock, Npas2, light, entrainment, mouse, phase-response curve, circadian rhythms
Introduction
In mammals, the circadian clock regulating behavioral rhythms is located in the suprachiasmatic nuclei (SCN) of the hypothalamus (reviewed in Weaver 1998). The SCN clock is re-set (entrained) by periodic stimuli, of which light is the most prevalent and potent. The circadian clock within the SCN is composed of a population of coupled, single-cell oscillators in which a transcriptional/ (post-)translational feedback loop operates (reviewed in Weaver and Reppert 2008). The transcriptional activation is provided by heterodimers of the transcription factors, CLOCK:BMAL1 or NPAS2:BMAL1. These heterodimers bind to E-box elements in the promoter region of the Period (Per) and Cryptochrome (Cry) genes, increasing their expression level. Upon translation and posttranslational modification, the protein products of these genes enter the nucleus in various complexes, interact with and inhibit the activity of the activator complex, and thereby suppress their own transcription. This molecular feedback loop has a cycle length of approximately 24 hours.
The CLOCK protein plays an important role in this molecular feedback loop. A mutant allele of the Clock gene was generated in a mutagenesis screen. Mice bearing one copy of this mutant allele have abnormally long (~ 24.5 hr) free-running period in constant darkness (DD), while homozygotes exposed to DD initially have very long period (~ 28 hr) and then become arrhythmic (Vitaterna et al. 1994). The mutation, located in an intron, leads to altered transcript splicing such that exon 19 is absent in the gene product (called CLOCKΔ19; King et al. 1997b). The CLOCKΔ19 protein retains its capacity to interact with binding partners and to bind to DNA, but the resulting complexes (e.g., with BMAL1) are deficient in functional activity (Gekakis et al. 1998; Jin et al. 1999). Studies of the mutant allele in combination with a large genomic deletion revealed that the CLOCKΔ19 product actively interferes with circadian rhythm generation (King et al., 1997a). Nevertheless, ClockΔ19/ Δ19 mice become rhythmic in constant light (Spoelstra et al. 2002), and the ClockΔ19/ Δ19 genotype retains circadian rhythmicity in DD on other genetic backgrounds (Kennaway et al. 2006; Ochi et al. 2003). Thus, additional mechanisms can provide positive drive to the circadian feedback loop. Because ClockΔ19/ Δ19 mice had abnormally long circadian period length regardless of background strain and lighting condition, however, it had been assumed that the Clock gene played a critical role in the function of the core oscillator.
DeBruyne et al. (2006) generated a null allele of Clock. Surprisingly, mice homozygous for this null allele (hereafter, Clock−/− or CLOCK-deficient mice) remain rhythmic in DD, with only a slight shortening of circadian cycle length (DeBruyne et al. 2006). NPAS2 is structurally similar to CLOCK, and is capable of heterodimerization with BMAL1 and driving E-box mediated gene expression (Hogenesch et al. 1998; Kume et al. 1999). Similar to CLOCK-deficient mice, NPAS2-deficient mice exhibit robust behavioral rhythms with a slightly shortened period in DD (DeBruyne et al. 2007a; Dudley et al. 2003). In the SCN, NPAS2 appears to be functionally redundant with CLOCK, because mice lacking both CLOCK and NPAS2 are arrhythmic (DeBruyne et al. 2007a). In peripheral tissues, however, CLOCK is necessary for rhythm maintenance (DeBruyne et al., 2007b)
While CLOCK and NPAS2 are functionally redundant in terms of rhythm maintenance in the SCN, mice lacking either protein apparently have abnormal responses to light. CLOCK-deficient mice have altered light resetting, with smaller phase delays after a 4-hour extension of the light phase and much larger phase advances after a 4-hour light pulse at the end of the night (DeBruyne et al. 2006). An advanced phase angle of entrainment to the lighting cycle also was observed in some experiments (DeBruyne et al. 2006; DeBruyne et al. 2007b). Npas2m/m mice also appear to have altered responses to light, as they re-entrain more rapidly than wild-type controls following a single 4-hour shift in the light/dark (LD) cycle (Dudley et al. 2003). Here, we more closely characterized the entrainment and phase resetting properties of CLOCK-deficient mice and, in some experiments, of Npas2m/m mice. The results reveal that CLOCK-deficient mice have profound abnormalities in circadian light responses in several paradigms.
Materials and Methods
Animals and Housing Conditions
Unless otherwise noted, male and female adult (3–12 month) mice were used. The genotypes examined were C57BL/6J (wild-type) and Clock−/− or Npas2m/m mice. The mutant alleles were backcrossed to the C57BL/6J background for at least 9 generations prior to generating homozygous animals for study. Generation of Clock−/− mice has been described elsewhere (DeBruyne et al. 2006). Founder mice used to establish our colony of Npas2m/m mice (Reick et al. 2001) were kindly provided by Dr. Steven McKnight (UT Southwestern, Dallas TX). Study mice were generated in our facility. Genotypes were determined using a PCR-based method, as previously described (DeBruyne et al. 2007a). All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School, and were in accordance with NIH guidelines and international standards.
At all times, animals were maintained in climate controlled closets at 21±1 °C and 30–60 % humidity, with food and deionized water available ad libitum. Animals in the general colony were group housed in individually ventilated mouse cages (191 × 279 × 127 mm).
For most wheel running experiments, animals were housed individually in ventilated microisolator cages (Allentown Inc., NJ, 393 × 285 × 194 mm) equipped with 125 mm diameter running wheels (DeBruyne et al., 2006). In some studies of constant light exposure, mice were housed in circular white cages (25 cm diameter) equipped with a light-emitting diode (LED) module in the cage roof. The LEDs (Tyntek China, 10 mm white ultra-bright LED) provide broad-spectrum illumination with a roughly Gaussian distribution of light across the range from 430–720 nm, with three embedded peaks typical of white LEDs. The circular cage design provided very even illumination across the cage, and different illumination levels could be achieved using a rheostatic dimmer switch and neutral density filters (Cinegel #3404, Rosco). Light intensities were measured with a VWR Traceable photometer. Running wheels in this cage type were 175 mm in diameter.
White light for lighting cycles was provided by fluorescent bulbs. The intensity of light is indicated for each experiment. Dim red light (>600 nm) was present at all times, except as noted. Records of lighting shown in the figures are data-derived, based on recording of the light present within each environmental closet using photoresistors and monitoring these as “light channels” in ClockLab software.
Phase Response Curve (PRC)
Twelve male mice of each genotype (Clock−/−, wild-type controls, and Npas2m/m) were habituated to running wheel cages for at least 2 weeks in 12L:12D. Then, animals were transferred to constant darkness (DD) for 15 weeks. During this time, animals received a 4-hour light pulse (400 lux) every 12 to 20 days at random times of day, i.e., an Aschoff type I protocol (Aschoff 1965). Free-running period was assessed in the 10 days before each light pulse. Each animal was exposed to 7 light exposures. The timing of light pulses and the resultant phase shifts were calculated and grouped in 2-hour circadian time (CT) bins (see Table S1 in Supplemental Online Material). In addition, a phase transition curve was calculated.
Phase shifts were compared using one-way repeated measures ANOVA with CT as within parameter and genotype as between parameter. Similarly, free-running periods were compared using one-way repeated measures ANOVA with Inter-light pulse interval as within parameter and genotype as between parameter. For both, Scheffé’s post-hoc test was used for comparisons between genotypes.
Entrainment to Skeleton Photoperiods
Wild-type (2 males, 3 females) and CLOCK-deficient (5 males, 9 females) mice were housed in microisolator cages with running wheels, and underwent baseline recording in 12L:12D (100 lux, lights on at 07:00). The light phase was then shortened to occur only during the final hour of the previous lighting cycle, e.g., lights on 18:00 – 19:00 (1L:23D). After 8 weeks in these conditions, a second light phase per 24-hr cycle was added to generate a cycle with lights on from 07:00 to 08:00 and from 18:00 to 19:00 (1L:10D:1L:12D).
Effects of Constant Light on Circadian Period
Two experiments were conducted. In the first, male mice (n = 9 per genotype) were entrained in LD (ca. 100 lux) in microisolator cages with running wheels for at least 2 weeks and then were released into constant light (LL, ca. 100 lux). After 23 days in LL, the lighting cycle was changed to DD. Free-running period was assessed for 10 consecutive days, beginning 5 days after placement in each lighting condition.
In an independent experiment, wild-type (10 male, 4 female) and Clock−/− (9 male, 5 female) mice were held in circular cages with illumination provided by LEDs. Red light was not present at any time. Light intensity levels were 0 lux (DD), 6 lux, 100 lux and 400 lux. Animals were tested in two groups, in a counterbalanced design. One group started in LD, followed by DD, and then by constant light with ascending intensities (6, 100, 400 lux). The second group started in LD, and then went to the brightest level of LL, and subsequently through descending light intensities (400, 100 and 6 lux, before DD). Each light level was presented for 14 to 20 days. Circadian period in each lighting condition was determined for 10 days, excluding the first 4–5 days after transfer to the new light intensity.
Free-running periods were compared using 2-way repeated measures ANOVA with light level as within parameter and group and genotype as between parameters. Scheffé’s post-hoc test was used where appropriate. Results from the groups differing in the order of treatments were not different and therefore were pooled.
Data Analysis
Running wheel data was recorded using ClockLab Data Collection (Actimetrics, Wilmette IL) and stored in 1 min bins. Actograms were double-plotted using ClockLab analysis program. Phase shifts, waveform of activity, onsets of activity, and free-running periods (τ) were calculated with ClockLab (Dallmann et al. 2007; DeBruyne et al. 2006). Data are reported as mean ± standard error of the mean (SEM). Statistical testing (described above for each data type) was performed using StatView 5.0. All statistical tests were 2-tailed and the level of significance was set to α = 0.05.
Results
Phase Angle of Entrainment in 12L:12D
We previously reported that CLOCK-deficient mice housed in an LD cycle were active for 2–3 hours before lights-out, but this abnormal behavior was seen inconsistently (DeBruyne et al. 2006). This premature activity, also called a positive phase angle of entrainment, can lead to ambiguity about the circadian phase of the oscillator in LD, and is one reason we performed the PRC studies (below) using an Aschoff type I design.
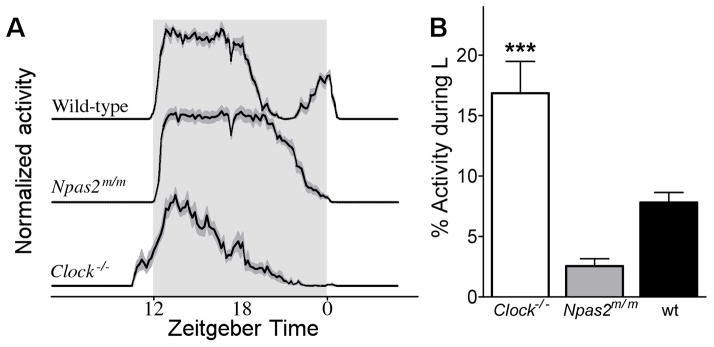
We first assessed phase angle of entrainment in male mice of three genotypes housed in 12L:12D. Clock−/− mice had a significantly larger portion of their daily activity during the light phase of the lighting cycle, relative to wild-type and Npas2m/m mice (Fig. 1). Plotting the activity profiles revealed that Clock−/− mice had an advanced phase angle of activity onset, compared to Npas2m/m and wild-type mice (Fig. 1A). On average, the onset of activity preceded lights-off by 1.2 ± 0.1 hrs in Clock−/− mice. In contrast, activity onset follows lights-off by 0.1 ± 0.1 hrs in Npas2m/m mice and by 0.0 ± 0.1 hrs in wild-type mice (n = 12 per genotype).
Figure 1.
(A) Average normalized waveform of activity of wild-type (top), Npas2m/m (middle), and Clock−/− mice (bottom) during the last 10 days of 12L:12D for the mice subsequently used for the PRC experiment. Shaded area signifies SEM around average (n = 12 per genotype). Grey box indicates LD schedule. (B) Quantification of the proportion of activity during the light phase for each genotype of the same animals and period as in A. *** indicates p < 0.001 vs. wild-type in post-hoc test.
Free-running period in DD
The male mice used to assess phase angle of entrainment were subsequently released into DD, and then were exposed to light pulses to construct a phase-response curve (see below). There was a significant effect of genotype on free-running period during the free-running portions of the PRC experiment (p < 0.001), but no change over the course of the experiment (p > 0.36). The average free-running periods (τDD) were 22.9 ± 0.2 hrs (Clock−/−), 23.4 ± 0.1 hrs (Npas2m/m) and 23.8 ± 0.1 hrs (wild-type). Clock−/− mice differed significantly from wild-type mice (p < 0.001), while a trend toward shorter τDD in Npas2m/m mice was not significant (p > 0.05), consistent with previous reports (DeBruyne et al., 2006, 2007b, Dudley et al, 2003).
Phase Response Curve (PRC)
Previous work showed that CLOCK-deficient mice had altered responses to a 4-hour light pulse on the first night of constant conditions (DeBruyne et al. 2006), i.e., in an Aschoff type II protocol (Aschoff 1965). This previous work examined only two phases, a 4-hour extension of the light phase of the lighting cycle (from ZT12–ZT16), and a pulse at ZT 20–24 (DeBruyne et al. 2006). To further characterize the light responses of CLOCK-deficient mice, we exposed mice to 4-hour light pulses at all circadian times while free-running in DD, i.e., an Aschoff type I protocol (Aschoff 1965). Four-hour light pulses were used because these give larger phase shifts than shorter light pulses, while the overall PRC shape is unaffected (Comas et al. 2006, 2007).
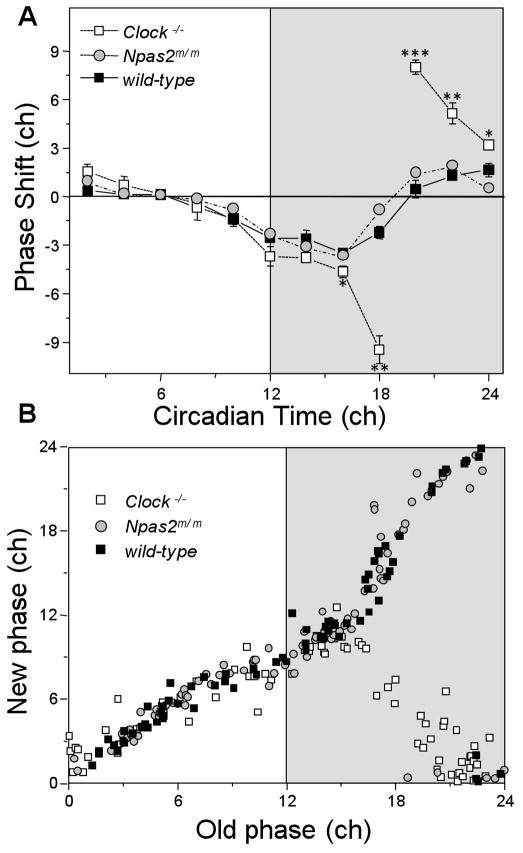
The magnitude of light-induced phase shifts for Npas2m/m mice was indistinguishable from wild-type mice at all times (Figs. 2 & 3). In contrast, Clock−/− mice had a qualitatively different PRC. Clock−/− mice showed phase shifts with a magnitude comparable to that of wild-type mice in response to light exposure during the subjective day and first 4 hrs of the subjective night, but very large phase shifts occurred in response to light applied in the middle and latter half of the subjective night, compared to wild type controls (Figs. 2 & 3). For statistical analysis, data were binned in 2-hour intervals based on the CT of the start of the light pulse. Clock−/− mice had significantly larger shifts than wild-type mice at CT16, 18, 20, 22, and 24 (Fig. 3A; see also Table S1). While the average phase shift following light exposure starting at CT 17–20 was ~ 9.5 hours, phase shifts of individual animals approached ~ 12 hrs. These very large phase shifts were immediate, without apparent “transient” days. For each genotype, very little change in free-running period was observed following 4-hr light pulses (data not shown), consistent with results of Comas et al (2006) for wild-type mice.
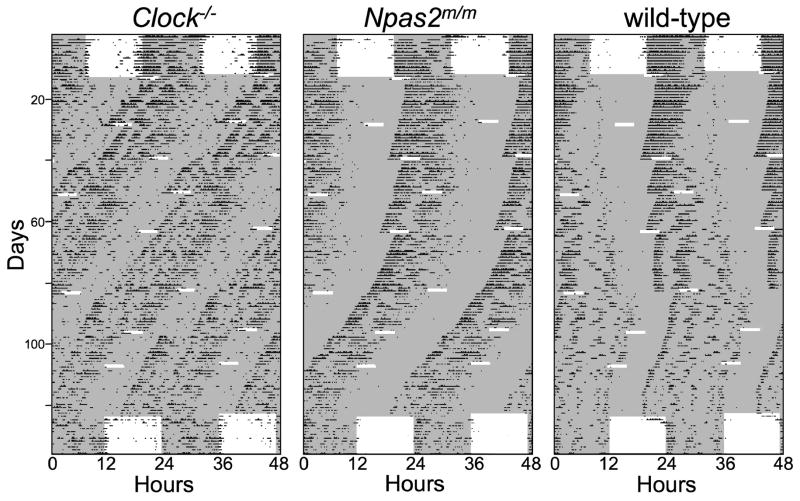
Figure 2.
Double plotted actograms of a representative Clock−/− (left), Npas2m/m (center) and wild-type (right) mouse during the PRC experiment. Shaded areas signify periods of darkness. The light pulse on the first day in DD was not included in the PRC because it was not preceded by a period of free-running rhythmicity in DD.
Figure 3.
(A) PRCs of Clock−/− (white squares), Npas2m/m (gray circles) and wild-type (black squares) mice, binned into 2-hour intervals. (B) Phase-transition curves for Clock−/− (white squares), Npas2m/m (gray circles) and wild-type (black squares) mice. Data for twelve mice and 76 to 86 pulses per genotype are depicted; see Supplemental Table S1 for details on number of pulses and values. Significant differences vs. wild-type are indicated as * p < 0.05, ** p < 0.01 and *** p < 0.001. Abbreviation: ch, circadian hours.
Phase shift data were also plotted as a phase-transition curve (Fig. 3B). The average slope of the plots were near one for wild-type (0.98; R2=0.93) and Npas2m/m (1.00; R2=0.90), respectively), indicating Type 1 resetting, while the average slope was near zero (0.05; R2=0.01) in Clock−/− mice (Type 0 resetting, Winfree 1980).
Two Clock−/− mice became arrhythmic in response to a 4-hour light pulse beginning near the middle of their circadian night (Fig. S1). In both cases, rhythmicity was reinstated by the next light pulse and their phase after the light pulse was similar. Arrhythmicity was not observed in mice of any other genotype, and could not be reproduced in the same animals with similarly timed light pulses given later in the experiment.
Due to the lack of phenotype of Npas2m/m mice with respect to phase angle of entrainment in 12L:12D and in the PRC experiment, they were not included in subsequent experiments.
Entrainment to Skeleton Photoperiods
To further assess the activity profile with less influence from acute effects of light on activity (i.e., masking), additional studies were performed using skeleton photoperiods. The skeleton lighting cycle used consisted of lighting during only the first and last hour of the previous light phase, while the half-skeleton photoperiod consists of only the last hour of light; these photocycles allow larger periods of time in darkness and thus allow assessment of activity profiles with reduced opportunity for masking (Pittendrigh and Daan 1976; Mrosovsky 1999).
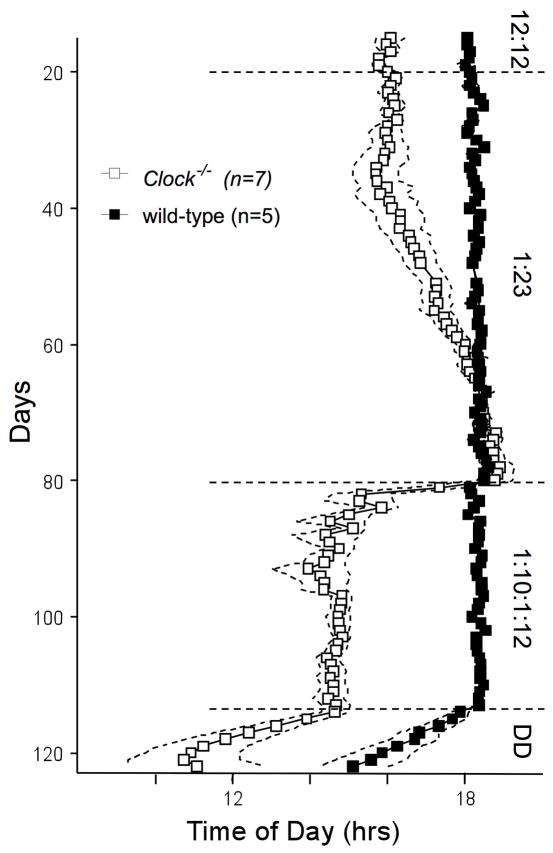
All wild-type mice remained entrained and started activity after the “evening” light pulse of the skeleton photoperiod, irrespective of the presence of the “morning” light pulse, indicating they are entraining by delays, as expected (Fig. 4). Seven (2 of 5 male and 5 of 9 female) Clock−/− mice transferred from 12L:12D to a half-skeleton photoperiod (1L:23D) behaved similarly to each other. These mice gradually delayed activity onset until activity began just after the 1 hr evening light phase, thus achieving a phase that was indistinguishable from wild-type mice (Figs. 4 & 5). Immediately after introduction of a second, 1-hr light phase each morning (1L:10D: 1L:12D), the advanced phase angle reappeared in Clock−/− mice (2.2 ± 0.1 hrs), but not in wild-type mice (−0.1 ± 0.1 hrs, Fig. 5). The magnitude of the positive phase angle was nearly twice as large in the 1L:10D:1L:12D skeleton photoperiod as in 12L:12D (Fig. 5; see also Fig. S2).
Figure 4.
Representative double-plotted actograms of a Clock−/− mouse and a wild-type mouse in the various light/dark cycles (12L:12D, 1L:23D, 1L:10D:1L:12D and DD). Shaded areas signify periods of darkness. A gap in data recording occurred from day 49 to day 51.
Figure 5.
Quantification of activity onset for Clock−/− and wild-type mice in various lighting conditions (12L:12D, 1L:23D and 1L:10D:1L:12D). Every data point represents the mean ± SEM (symbol ± dashed lines) activity onset of 7 Clock−/− (2 males and 5 female; open symbols) and 5 wild-type (closed symbols) mice on one day.
The response of Clock−/− mice to the half-skeleton (1L:23D) photoperiod was heterogeneous, however. Seven of the mice behaved similarly, as described above. Of the 7 remaining mice, 4 did not entrain to 1L:23D by delays, but instead advanced their activity onsets ‘around the clock’ until they locked on to the 1-hour light phase, with activity onset now coordinated to the end of the light. Two Clock−/− mice did not entrain to the half-skeleton photoperiod, while one mouse initially free-ran with a short period, and then showed relative coordination to the light pulse, with an advanced phase angle of entrainment. Because these 7 mice took much longer to establish a stable phase angle, and their activity onsets were very different from the group of mice exemplified in Fig. 4, and from each other, these mice were excluded from the analysis presented in Fig. 5. Five of these seven ‘atypical’ Clock−/− mice rapidly assumed the advanced phase angle of entrainment typical of Clock−/− mice when a second 1-hr light pulse per cycle was introduced (1L:10D:1L:12D; data not shown).
An independent, slightly shorter experiment with a different order of lighting schedules was conducted; the overall pattern of results was very similar (Fig. S2).
Overall, the PRC and skeleton photoperiod studies suggest that the advanced phase angle of entrainment of Clock−/− mice in 12L:12D is due to a very strong phase-advancing effect of light in the morning, which cannot be overcome by the more modest phase delaying effects of light in the evening.
Effects of Constant Light on Circadian Period
Constant light (LL) affects circadian period in mice (Aschoff 1952). Like most nocturnal rodents, wild-type mice have a larger phase delay portion of the PRC than phase advance portion, and constant light, by falling on both portions, leads to an increase in period length. In view of the abnormally large phase advance portion of the PRC of Clock−/− mice, we predicted that CLOCK-deficient mice would not lengthen period when exposed to LL.
In the first LL experiment, we examined period length at a single light intensity (100 lux) and compared it toτDD. The τDD of these Clock−/− mice was 0.7 ± 0.2 hrs shorter than that of the control mice, confirming previous results (DeBruyne et al., 2006; 2007b, DD results from the PRC study, above). The free-running period of wild-type controls was increased in LL by 0.5 ± 0.1 hrs (p < 0.001) compared to DD (τLL: 24.4 ± 0.1 hrs vs. τDD: 23.9 ± 0.1 hrs). In contrast, and consistent with our expectations, the free-running period of Clock−/− mice in LL was 0.3 ± 0.2 hrs shorter than in DD (p < 0.05; τLL: 22.9 ± 0.1 hrs vs. τDD: 23.3 ± 0.2 hrs).
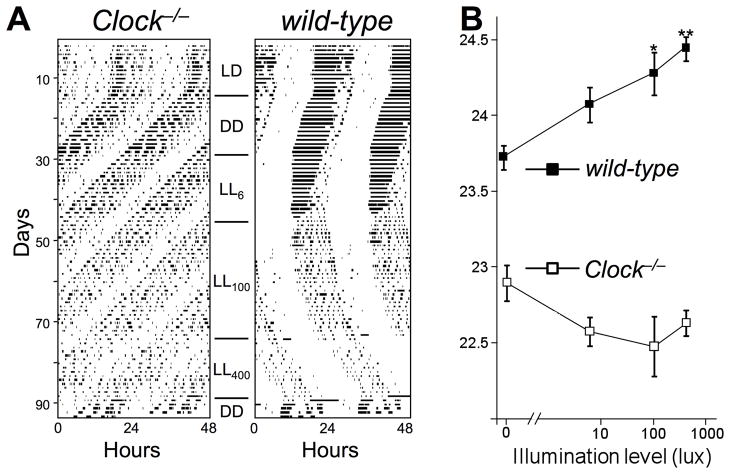
Since lengthening of period in constant light is typically dependent on light intensity in mice (Aschoff 1960), we next tested LL at multiple illumination levels. In this second experiment, LED light sources and circular white cages were used for better control over illumination levels. Again, the average free-running period of wild-type controls increased significantly with increasing light intensities (p < 0.01). In contrast, the free-running period of Clock−/− mice did not increase with light intensity, but in fact tended to be shorter in constant light, independent of intensity (ANOVA: p > 0.05, n.s.) (Fig. 6). One potential explanation for the absence of a lengthening effect of constant light on circadian period would be insensitivity to light. Notably, however, LL inhibited locomotor activity levels in an intensity-dependent manner in both CLOCK-deficient and wild-type mice (Fig. S3).
Figure 6.
(A) Representative double-plotted actograms of Clock−/− and wild-type mice in constant light (LL) of different intensities. (B) Quantification of free-running period in LL of different intensities in Clock−/− and wild-type mice (n = 14 each). In wild-type mice, period increased significantly with illumination level (p < 0.01), while illumination level had no significant effect in Clock−/− mice. Post-hoc testing revealed that period length of wild-type mice was significantly increased at 100 lux (* p < 0.05) and at 400 lux (** p < 0.01), relative to DD, but was not different at 6 lux (p > 0.05).
Discussion
Compared to the very marked phenotype of ClockΔ19/Δ19 mice in DD, characterized by a very long (~28 hr) period and decay into arrhythmicity (Vitaterna et al. 1994), CLOCK-deficient mice have only a mild phenotype in DD, exhibiting a slightly shorter free-running period (DeBruyne et al. 2006, present results). Nevertheless, our earlier study indicated that circadian responses to light are altered in the absence of CLOCK (DeBruyne et al. 2006). In the present work, we have thoroughly investigated the responses of CLOCK-deficient mice to light, identifying a number of significant abnormalities that appear to be functionally interrelated. CLOCK-deficient mice exhibit much larger phase advances to light exposure during late night / early morning than wild-type mice, which is evident in the Type 0 PRC to 4-hour light pulses. Together with a slightly shorter endogenous period, large phase advances to light might be responsible for both the altered phase angle of entrainment in LD and the absence of LL-induced lengthening of period (see below).
To test the hypothesis that morning light had a profound impact on entrainment in CLOCK-deficient mice, we subjected the mice to skeleton photoperiods. In the half-skeleton photoperiod (1L:23D) with only 1 hr of light in the evening and no light in the morning, the animals gradually entrained to the same phase as wild-type mice (Figs. 4 & 5). When the morning light was reintroduced (1L:10D:1L:12D), the Clock−/− mice regained their advanced phase angle almost immediately. These data strongly suggest that light in the morning does indeed cause a significant advance of the oscillator and of activity. The very slow time-course by which Clock−/− mice phase delay to the wild-type-like phase angle in the half-skeleton photoperiods is somewhat surprising, as it contrasts with the rapid response to single light pulses in the PRC experiment. This slow response may reflect conflicting influences related to the relatively small phase delay zone of the PRC and the slightly shorter free-running period of the CLOCK deficient mice. Indeed, several animals entrained to the half-skeleton photoperiod by advances rather than by delays.
Detection of an advanced phase angle of entrainment in the running wheel records of CLOCK-deficient mice housed in 12L:12D requires – by definition – that the mice use the running wheel during the light phase of the light-dark cycle. Light is, however, a potent inhibitor of wheel-running activity in mice, an effect referred to as ‘negative masking’ (Mrosovsky 1999). Thus, we previously proposed that Clock−/− mice might have an alteration in masking (DeBruyne et al. 2006). Altered masking has been described for ClockΔ19/Δ19 mice (Redlin et al. 2005), but masking has not been examined in detail in CLOCK-deficient mice. While a change in masking may allow the expression of the advanced phase angle in 12L:12D, the expression of a similarly advanced phase angle of entrainment in skeleton photoperiods indicates that possible alterations in masking are not causally related to the advanced phase angle phenotype. The free-running period of CLOCK-deficient mice is 0.5–0.7 hr shorter than in wild-type mice. This period difference may contribute to the advanced phase angle of entrainment in CLOCK-deficient mice, but it seems unlikely to account for the entire ~ 1.5–2 hr advance in activity onset.
Another possible explanation for the altered phase angle of entrainment and altered entrainment in skeleton photoperiods in CLOCK-deficient mice is an increase in the duration of the nocturnal photosensitive period. As a proxy for this, we calculated activity duration (alpha) for all animals used for the PRC, for a 10-day period while the mice were free-running in DD. Manual calculation was performed because alpha in mice is quite variable and difficult to determine. We found no significant difference among the genotypes (data not shown).
“Aschoff’s rule” summarizes the observation that nocturnal rodents increase their free-running period with increasing intensity of constant light. This is attributed to the larger phase-delay portion of the PRC, relative to the phase advancing portion, so that when illumination falls on the entire circadian cycle, progressive delay of the oscillator results. CLOCK-deficient mice defy Aschoff’s rule by not lengthening their free-running period in LL. In both experiments (one comparing DD with LL of ca. 100 lux, and another examining 3 light at 6, 100, and 400 lux), period length of wild-type mice increased in LL, while CLOCK-deficient mice did not. This may result from the larger phase advance region of the PRC in CLOCK-deficient mice; illumination falling throughout the circadian cycle advances the oscillator. It is worth noting, however, that the mechanistic relationship between LL-induced period lengthening and the PRC is far from clear, as exemplified by Syrian hamsters, which have a larger phase advance zone of the PRC and yet show intensity-dependent period lengthening in LL.
The propensity of CLOCK-deficient mice to be active during the afternoon when housed in an LD cycle, and the failure of CLOCK-deficient mice to lengthen period in constant light could result from difficulty perceiving light. Several lines of evidence indicate that this is not the case. First, the PRC data indicate that CLOCK-deficient mice actually have larger phase shifts when light exposure occurs in the middle or late subjective night. Second, LL inhibited activity levels in an intensity-dependent manner in both CLOCK-deficient and wild-type mice (Fig. S3), suggesting no gross defect in negative masking. Finally, we examined light-induced c-fos expression in the SCN at CT13, and found robust light-induced expression (relative to dark controls) that did not differ between the genotypes (Fig. S4). These data directly demonstrate the functional integrity of the retino-hypothalamic tract. Nevertheless, we cannot exclude subtle differences in sensitivity to light. In fact, the retinal clock can influence light perception (Guido et al. 2010; Weng et al. 2009). While Clock−/− mice do not have functional peripheral clocks (DeBruyne et al. 2007b), their retinal clock has not yet been examined.
Mice with mutations in other circadian clock genes resemble some aspects of the phenotype of CLOCK-deficient mice, but no model fully reproduces it. Per2Brdm1 mice have a short period in DD, but become arrhythmic with extended time in DD (Zheng et al., 2001), and the PRC is Type 1 (Spoelstra et al. 2004). PER2 has been proposed to play an important role in period lengthening in LL (Munoz et al., 2005), and Per2Brdm1 mice do not lengthen free-running period in LL (Steinlechner et al. 2002). Rev-erbα−/− mice have a slightly shorter period in DD, large phase advances in response to light late in the night, and their period lengthening response to LL is blunted but not absent (Preitner et al. 2002; Jud et al., 2010). Mice lacking both PER1 and REV-ERBα maintain rhythmic Per2 expression and behavioral rhythmicity, have short a period, have only modest increase in τLL and have a type 0 PRC (Jud et al. 2010), and thus are most similar to Clock−/− mice. Molecular deficits in the SCN of CLOCK-deficient mice include a modest reduction in peak PER2 levels, great reductions in Rev-erbα and Per1 expression, and reduction in BMAL1 and PER1 protein levels to ~10 % of the wild-type peak level (DeBruyne et al. 2006). This constellation of molecular deficits may contribute to reduction in pacemaker amplitude that underlie the altered PRC and type 0 resetting in CLOCK-deficient mice, with only subtle alterations in free-running period in DD. Collectively, these studies reveal that τDD, τLL, and circadian responses to light can be differentially affected by molecular lesions.
CLOCK and NPAS2 are functional homologs and are critically important for circadian clock function (DeBruyne et al. 2007a). Like Clock−/− mice, Npas2m/m mice have a slightly shorter τDD compared to wild-type animals (Dudley et al. 2003), although this difference has not been statistically significant in our studies (DeBruyne et al., 2007a; present results). Npas2m/m mice re-entrain more rapidly than wild-type mice following a 4-hr advance of the lighting cycle (Dudley et al. 2003). In apparent contrast with the results of Dudley et al. (2003), however, we found that Npas2m/m mice have a wild-type-like PRC (Figs. 1 & 2). Re-entrainment to a phase-shifted lighting cycle may be a more complicated assay of circadian light responses, or the shorter free-running period might be responsible for faster re-entrainment. We also found that Npas2m/m mice have a normal phase angle of entrainment in an LD cycle. The much more marked abnormalities in photic responses of Clock−/− mice, relative to Npas2m/m mice, indicate that CLOCK plays an important role for which NPAS2 cannot substitute. With respect to light resetting, NPAS2 is not able to fully rescue a CLOCK-less clock.
In ClockΔ19/+ mice, it has been proposed that the type 0 PRC in response to 6-hour light pulses is due to reduced pacemaker amplitude (Vitaterna et al. 2006). On a molecular level, this is reflected by lower amplitude Per1 rhythmicity coupled with normal induction of Period gene expression by light (Vitaterna et al. 2006). In the SCN of Clock−/− mice, the rhythmic expression of several clock genes and output genes is dampened at the RNA and protein levels (DeBruyne et al. 2006; see above). Therefore, it is possible that type 0 resetting in CLOCK-deficient mice is due to reduction in circadian oscillator amplitude, as proposed for ClockΔ19/+ mice.
In Clock−/− mice, enhanced resetting may arise by another mechanism, distinct from a global reduction in the amplitude of single-cell oscillators. In the SCN of CLOCK-deficient mice, there is a drastic (~90%) reduction in the number of neurons immuno-positive for BMAL1, suggesting that the complement of functional circadian oscillators is reduced by 90% (DeBruyne et al. 2006). The remaining 10% of neurons are nevertheless sufficient to maintain rhythmicity in SCN explants and to maintain behavioral rhythms, due to intercellular coupling (DeBruyne 2008; Liu et al. 2007). In contrast, peripheral tissues cannot maintain coherent rhythmicity in vitro, likely because they lack coupling (DeBruyne et al. 2007b). Models of locally coupled oscillators can produce type 0 resetting if coupling strength is reduced (Achermann and Kunz 1999). A smaller population of functional oscillators will have an inherently lower degree of oscillator coupling, making it easier to reset to a different phase compared to a whole network of functional oscillators. Thus, reduction in the number of functional SCN oscillators, and the resulting decrease in their coupling strength, may contribute to the type 0 PRC in Clock−/− mice.
Whether amplitude reduction occurs by reduction in individual oscillator amplitude or by reduction in the number of functionally coupled oscillators, amplitude reduction is likely key to understanding the light-resetting phenotype of CLOCK-deficient mice. Theory on PRC’s indicates that reduced pacemaker amplitude predisposes the oscillator for type 0 resetting (Johnson 1999; Johnson et al., 2003). Furthermore, when a strong input stimulus induces a large phase shifts in a limit-cycle oscillator, both phase and amplitude of the oscillator are affected (Winfree 1980). In Clock−/− mice, reduced initial oscillator amplitude may make the oscillator very sensitive to the amplitude-reducing effects of a light pulse, relative to wild-type mice. Amplitude is likely not constant throughout the cycle, and a stimulus-induced reduction in amplitude may have greater effect when initial amplitude is lower, leading to a type 0 PRC, and explaining the phase-specific alteration of the PRC. Furthermore, light-induced reduction in pacemaker amplitude likely explains the light pulse-induced arrhythmicity observed in two cases (Fig. S1); appropriately timed light drives pacemaker amplitude to zero. Additional studies with shorter duration or lower intensity light could support this speculation, if a type 1 response were observed to less intense stimuli. Further molecular and physiological analysis of genetically modified mouse models will be needed to define the key biochemical substrates and mechanisms that define pacemaker amplitude.
Supplementary Material
Acknowledgments
We thank Chris Lambert and Liz Yu for technical assistance, Premananda Indic, Steve Reppert, and Bill Schwartz for comments on an earlier version of this manuscript, and S.L. McKnight for providing Npas2m/m founder mice used to establish our colony. This work was supported by the National Institutes of Health (awards R01 NS056125 to DRW and NRSA F32 GM074277 to JPD) and by the Deutsche Forschungsgemeinschaft (DFG, award DA525/2-1 to RD). Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring agencies.
References
- Achermann P, Kunz H. Modeling circadian rhythm generation in the suprachiasmatic nucleus with locally coupled self-sustained oscillators: phase shifts and phase response curves. J Biol Rhythms. 1999;14:460–468. doi: 10.1177/074873099129001028. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Changes of frequency of periods of activity of mice in constant light and lasting darkness. Pflugers Arch. 1952;255:197–203. doi: 10.1007/BF00363483. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor Symposia on Quantitative Biology. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian Clocks. North-Holland; Amsterdam: 1965. pp. 95–111. [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–372. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Circadian response reduction in light and response restoration in darkness: a “skeleton” light pulse PRC study in mice (Mus musculus) J Biol Rhythms. 2007;22:432–444. doi: 10.1177/0748730407305728. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Lemm G, Mrosovsky N. Towards easier methods in studying non-photic entrainment in mice. J Biol Rhythms. 2007;22:458–61. doi: 10.1177/0748730407306042. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP. Oscillating perceptions: the ups and downs of the CLOCK protein in the mouse circadian system. J Genetics. 2008;87:437–446. doi: 10.1007/s12041-008-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007a;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007b;17:R538–539. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Guido ME, Garbarino-Pico E, Contin MA, Valdez DJ, Nieto PS, Verra DM, Acosta-Rodriguez VA, de Zavalia N, Rosenstein RE. Inner retinal circadian clocks and non-visual photoreceptors: novel players in the circadian system. Prog Neurobiol. 2010;92:484–504. doi: 10.1016/j.pneurobio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Johnson CH. Forty years of PRCs--what have we learned? Chronobiol Int. 1999;16:711–743. doi: 10.3109/07420529909016940. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- Jud C, Hayoz A, Albrecht U. High amplitude phase resetting in rev-erbalpha/per1 double mutant mice. PLoS One. 2010;5:e12540. doi: 10.1371/journal.pone.0012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Owens JA, Voultsios A, Varcoe TJ. Functional central rhythmicity and light entrainment, but not liver and muscle rhythmicity, are Clock independent. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1172–1180. doi: 10.1152/ajpregu.00223.2006. [DOI] [PubMed] [Google Scholar]
- King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, Turek FW, Takahashi JS. The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Genetics. 1997a;146:1049–1060. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian Clock gene. Cell. 1997b;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Masking: history, definitions and measurement. Chronobiol Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- Munoz M, Peirson S, Hankins MW, Foster R. Long-term constant light induces constitutive elevated expression of mPER2 protein in the murine SCN: A molecular basis for Aschoff’s rule? J Biol Rhythms. 2005;20:3–14. doi: 10.1177/0748730404272858. [DOI] [PubMed] [Google Scholar]
- Ochi M, Sono S, Sei H, Oishi K, Kobayashi H, Morita Y, Ishida N. Sex difference in circadian period of body temperature in Clock mutant mice with Jcl/ICR background. Neurosci Lett. 2003;347:163–166. doi: 10.1016/s0304-3940(03)00688-8. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents - I. The stability and lability of spontaneous frequency. J Comp Physiol. 1976;106:223–252. [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Redlin U, Hattar S, Mrosovsky N. The circadian Clock mutant mouse: impaired masking response to light. J Comp Physiol A. 2005;191:51–59. doi: 10.1007/s00359-004-0570-z. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Spoelstra K, Albrecht U, van der Horst GT, Brauer V, Daan S. Phase responses to light pulses in mice lacking functional per or cry genes. J Biol Rhythms. 2004;19:518–529. doi: 10.1177/0748730404268122. [DOI] [PubMed] [Google Scholar]
- Spoelstra K, Oklejewicz M, Daan S. Restoration of self-sustained circadian rhythmicity by the mutant Clock allele in mice in constant illumination. J Biol Rhythms. 2002;17:520–525. doi: 10.1177/0748730402238234. [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Jacobmeier B, Scherbarth F, Dernbach H, Kruse F, Albrecht U. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J Biol Rhythms. 2002;17:202–209. doi: 10.1177/074873040201700303. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. Circadian Timekeeping. In: Squire LR, et al., editors. Fundamental Neuroscience. 3. Elsevier / Academic Press; Amsterdam ; Boston: 2008. pp. 931–958. [Google Scholar]
- Weng S, Wong KY, Berson DM. Circadian modulation of melanopsin-driven light response in rat ganglion-cell photoreceptors. J Biol Rhythms. 2009;24:391–402. doi: 10.1177/0748730409343767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree AT. The Geometry of Biological Time. Springer Verlag; New York: 1980. [Google Scholar]
- Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1990;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.