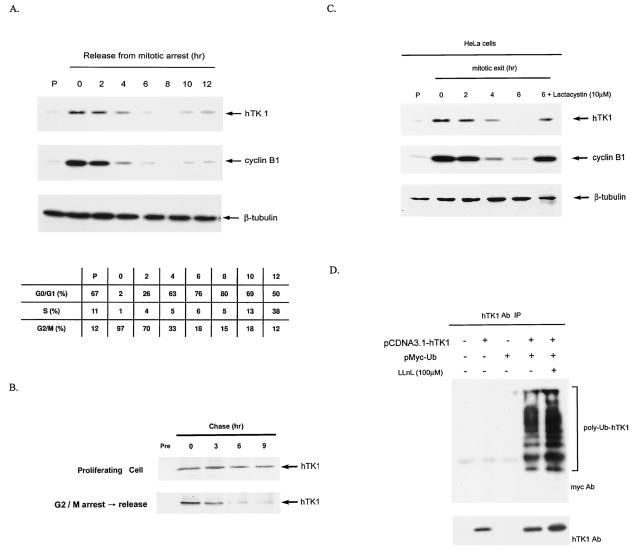
FIG. 1.
Mitotic degradation of hTK1 is via the ubiquitin-proteasomal pathway. (A) HeLa cells were arrested by nocodazole treatment for 20 h, followed by replenishment of fresh medium to release cells from the G2/M arrest. Extracts were prepared from HeLa cells released from G2/M arrest at the time points as indicated. The extract from asynchronized cells is indicated as the proliferating state (P). Expression of hTK1, cyclin B1, and β-tubulin was analyzed by Western blotting with anti-hTK1, anti-cyclin B1, and anti-β-tubulin antibodies, respectively. The cell cycle profile of corresponding cells was analyzed by flow cytometry as described in Materials and Methods and is expressed in the inset tables as percentages of cells in different phases. (B) HeLa cells of proliferating state or releasing from G2/M arrest were metabolically labeled with [35S]methionine (250 μCi/ml) for 30 min, followed by addition of fresh medium at the indicated time points prior to cell lysis. Cell extracts were immunoprecipitated by anti-hTK1 antibody, and the immunocomplexes were resolved by SDS-PAGE (12% polyacrylamide), followed by autoradiography. Normal rabbit immunoglobulin G (Pre) was used as a negative control. (C) Extracts were prepared from HeLa cells released from G2/M arrest. Cells were treated with lactacystin (10 μM) for 6 h during the mitotic exit phase. (D) LM-TK− cells were transfected with (+) or without (−) pCDNA3.1-hTK1 and pMyc-Ub as indicated. hTK1 was immunoprecipitated (IP) with anti-hTK1 antibody (Ab) and immunocomplexes were analyzed by SDS-PAGE and Western blotting with anti-hTK1 and anti-Myc antibodies.