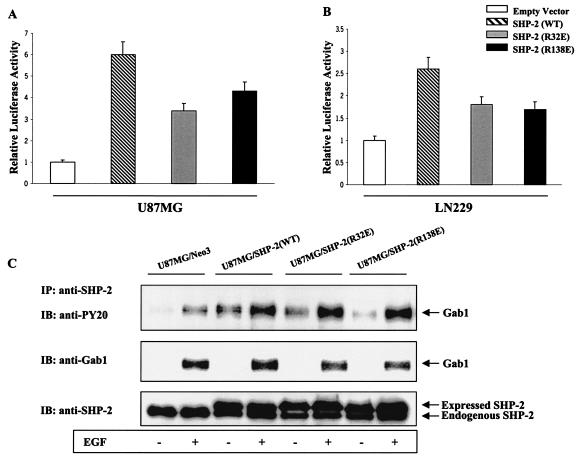
FIG. 8.
Reduced NF-κB activity in glioblastoma cells expressing SH2 domain mutants of SHP-2. (A and B) U87MG and LN229 cells were cotransfected with the 5× NF-κB-Luciferase promoter, the pSV-β-galactosidase vector, and 0.5 μg of vectors expressing either one of the two SH2 domain mutant of SHP-2: R32E (N-SH2) or R138E (C-SH2). Forty-eight after transfection, cells were placed in serum-free medium for 24 h followed by cell lysis. Cell lysates were assayed for luciferase and β-galactosidase activities according to the manufacturer's instruction (Promega). Luciferase values were normalized by β-galactosidase activities from three different experiments. The data are represented as means ± standard deviation, considering relative luciferase activity of cells transfected with empty vector as 1. P values were <0.05. (C) U87MG cells were stably transfected with either empty vector or vectors independently expressing wild-type [SHP-2(WT)], SHP-2(R32E), or SHP-2(R138E) cDNAs, serum starved for 24 h, and incubated with or without EGF (50 ng/ml) for 5 min. Equal amounts of whole lysates from each sample were subjected to immunoprecipitation by anti-SHP-2 antibody followed by SDS-PAGE and immunoblotting with antiphosphotyrosine and anti-Gab1 antibodies. The membrane was stripped and reprobed with anti-SHP-2 antibody to confirm consistent immunoprecipitation of SHP-2.