Figure 3.
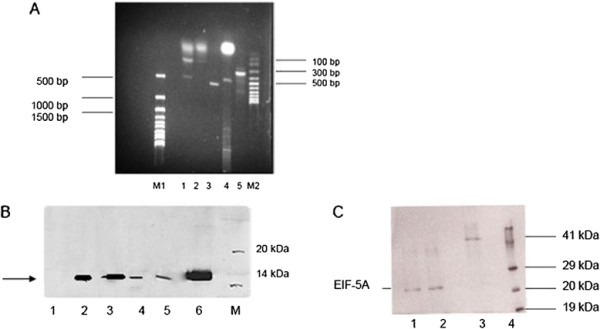
A) Monitoring in vivo silencing of parasitic eIF-5A by RT-PCR in RBCs of infected NMRI mice 2 days post infection. NMRI mice were infected with transgenic schizonts harbouring the expressed shRNA P#18. M1) 1 kb ladder (LifeTechnologies, Karlsruhe, Germany); 1) non-transfected 293T cells 2) EIF-5A-siRNA; 3) A positive control for the quality of cellular RNA is the 548 bp amplificate generated with α-tubulin gene-specific primers from P. berghei; 4) A PCR-control reaction with eIF-5A-gene specific primers from P.vivax generates a 448 bp cDNA fragment; 5) Amplificate of 323 bp obtained in a RT-PCR control reaction from a 1.4 kb kanamycin mRNA; M2) 100 bp ladder (Life Technologies, Karlsruhe, Germany). B) Western Blot analysis of parasitic sh-eIF-5A expression from transgenic schizonts after infection of NMRI mice. Protein extracts were generated from: 1) shEIF-5A RNA #P18; 2) shDHS-RNA; #P176; 3) protein extract from RBCs infected with P. berghei ANKA strain and 4) mock strain (without transfected shRNA); 5 and 6) different protein concentrations of the EIF-5A histidine-tagged, purified protein. 7) Standard protein marker (Roth). Polyclonal-antibody against EIF-5A protein from P. vivax was applied in a concentration of 1:1000 which detected the protein band with a molecular weight of 20 kDa. The protein concentrations of the extracts were 10 μg/μl. C) Confirmation of the specificity of the used anti-eIF-5A antibody by Western Blot analysis. 1) Protein extract prepared from NMRI infectected mice expressing the #18 eIF-5A-specific shRNA, supplemented with recombinant eIF-5A protein from P.vivax; 2) purified, recombinant EIF-5A protein from P.vivax; 3) Protein extract prepared from NMRI infected mice expressing the #18 eIF-5A-specific shRNA. The protein concentration was 10 μg in each lane.
