Figure 2 .
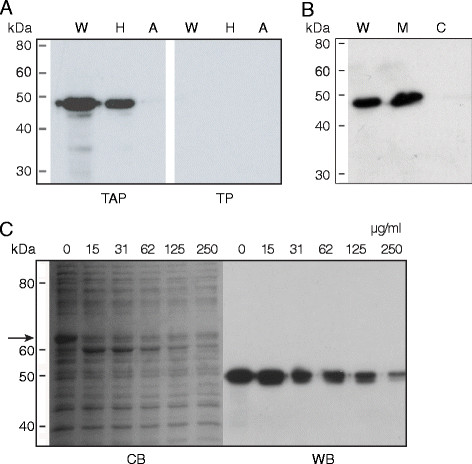
Immuno-detection of PhoA in fractionated or trypsin treated cellular proteins.A. Triton X-114 partitioning of M. gallisepticum cell proteins. Proteins of pTAP or pTP transformed cells were separated into hydrophobic and aqueous fractions by Triton X-114 partitioning, Western transferred and probed with a MAb to alkaline phosphatase. Panel TAP, M. gallisepticum transformed with pTAP and expressing PhoA. Panel A, M. gallisepticum transformed with pTP cells. Lanes W, whole-cells; H, hydrophobic fraction; A, aqueous fraction. B. Immunostaining of cytosolic and membrane fractions of mycoplasma transformants expressing alkaline phosphatase. The fractions were separated on 10 % SDS-polyacrylamide gels, Western transferred and immunostained using a MAb to alkaline phosphatase. Lanes W, whole cells; M, membrane fraction and C, cytosolic fraction. C. Surface proteolysis of PhoA. Whole pTAP transformant cells were treated with increasing concentrations of trypsin, the proteins then separated on 10 % SDS-polyacrylamide gels, Western transferred and immunostained using a MAb to AP. Trypsin concentrations (μg/ml) are indicated above each lane. Panels CB, Coomassie brilliant blue stained; WB, Western blot probed with MAb to AP. The arrow indicates the 67 kDa VlhA, which was degraded by increasing concentrations of trypsin. The tryptic products of VlhA can also be seen. Most cellular proteins were minimally affected.
