FIG. 3.
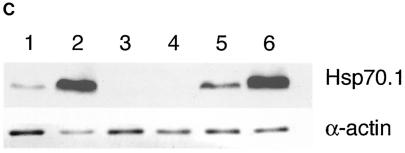
Synthesis of Hsp70.1 and Hsp70.3 mRNA and protein in Hsp70.1/3−/− and Hsp70.1/3+/+ MEFs. (A) Northern blot analysis of Hsp70.1 and Hsp70.3 mRNA levels. Cells were subjected to heat shock at 43°C for 30 min and then examined for mRNA expression of Hsp70.1 and Hsp70.3 with a radiolabeled DNA fragment probe derived from the mouse Hsp70.1 or Hsp70.3 gene coding region. (a) Control (Hsp70.1/3+/+) cells treatedwith heat and examined for mRNA expression. Lane 1, control RNA from unheated cells; lanes 2 to 5, cells subjected to heat shock and recovery for 1, 4, 8, and 24 h, respectively; lanes 6 to 9, cells subjected to heat shock, recovery for 24 h, heat treatment again for 30 min at 43°C, and no recovery or 1, 4, or 8 h of recovery, respectively. Note that the primary MEFs do not show detectable levels of Hsp70.1 and Hsp70.3 under nonstress conditions and that the heat shock induces both Hsp70.1 and Hsp70.3 mRNA. The induction of Hsp70.1 and Hsp70.3 is restored after a second heat shock treatment. (b) Mutant (Hsp70.1/3−/−) and control (Hsp70.1/3+/+) cells treated with heat and examined for mRNA expression. Lanes 1 to 7, Hsp70.1/3−/− cells; lane 1, control RNA from unheated cells; lanes 2 to 5, cells subjected to heat shock and no recovery or 1, 4, or 24 h of recovery, respectively; lanes 6 and 7, cells subjected to heat treatment, recovery for 24 h, heat treatment again for 30 min at 43°C, and no recovery or 2 h of recovery, respectively; lanes 8 and 9, Hsp70.1/3+/+ cells subjected to heat shock with either no or 1 h of recovery, respectively. The induction of Hsp70.1 and Hsp70.3 is restored in Hsp70.1/3+/+ but not in Hsp70.1/3−/− cells after a second heat shock treatment. (B) Heat-induced synthesis of Hsp70 in fibroblasts with and without Hsp70.1/3 genes. Polyacrylamide gel analysis of 3H-leucine-pulse-labeled (1 h) proteins synthesized after heat shock treatment. Shown is protein synthesis in Hsp70.1/3+/+ and Hsp70.1/3−/− cells (a) and Hsp70.1/3−/− cells without and with ectopically expressing Hsp70.1 (b). Cells were subjected to heat shock at 43°C for 30 min and no further treatment (lane 1); or recovery for 24 h (lane 2); or recovery for 24 h and a second heat shock at 43°C for 30 min (lane 3) or recovery for 1 h (lane 4). Note the appearance of the 70-kDa protein in lanes 2 and 4 representing cell lysates of Hsp70.1/3+/+ and Hsp70.1/3−/− cells with ectopically expressed Hsp70.1. The arrow indicates the appearance of the 70-kDa protein in Hsp70.1/3+/+ and Hsp70.1/3−/− cells with ectopically expressed Hsp70.1, while no such band is found in Hsp70.1/3−/− cells. (C) Western blot analysis for HSP70 synthesis following heat shock as detected by anti-HSP70 antibody. Cells were subjected to heat shock at 43°C for 30 min (with 2 h of recovery in lanes 2, 4, and 6) and examined for HSP70 protein with anti-HSP70 antibody. Lanes: 1 and 2, Hsp70.1/3+/+ cells; 3 and 4, Hsp70.1/3−/− cells; 5 and 6, Hsp70.1/3−/− cells with ectopically expressed Hsp70.1. Note the increase in HSP70 protein in lanes 2 and 6 whereas no HSP70 was detected in lanes 3 and 4.