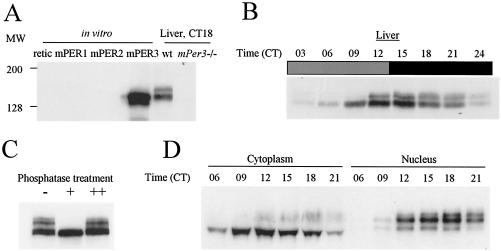
FIG. 1.
Rhythmic posttranslational modification of mPER3. (A) Characterization of antisera against mPER3. Equal amounts of in vitro translated mPER1, mPER2, mPER3, and reticulolycyte lysate were resolved by SDS-PAGE, along with liver extracts from wild-type (wt) and mPer3-deficient mice. The proteins were transferred onto nitrocellulose membrane and Western blotted. Representative Western blots with one of our strongest antisera (P3-28R) are shown. The antibody only recognized in vitro-translated mPER3 and not equal amounts of mPER1 or mPER2. Anti-mPER1 (PER1-1-R) and -mPER2 (PER2-1-R) antibodies only recognized in vitro-translated mPER1 and mPER2, respectively (data not shown). Similar sized bands were detected only in liver extracts from wild-type mice and not mPer3-deficient mice. (B) Circadian rhythms of mPER3. Liver extracts prepared from mice collected at 3-h intervals were Western blotted with P3-28R and anti-β-actin antibodies. Even loading of total protein was assured by Western blot with anti-β-actin antibody (data not shown). (C) Electrophoretic mobility shift of mPER3 is due to phosphorylation. Liver extract from CT15 was subjected to IP with anti-mPER3 antibody (P3-28GP). The immune complexes were split into three aliquots and incubated in the presence (+) or absence (−) of λPP or with both λPP and 40 mM vanadate (++). (D) mPER3 exhibits nuclear accumulation in a circadian manner. Mouse liver extracts were collected at the indicated times, fractionated into cytoplasmic and nuclear portions, and blotted for mPER3. Both cytoplasmic and nuclear fractions for each time point were derived from the same number of cells.