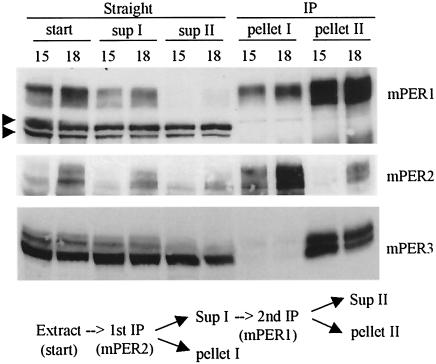
FIG. 5.
The mPER1-mPER3 complex is more abundant than the mPER2-mPER3 complex. Liver extracts from CT15 and CT18 were subjected to sequential IP reactions. Antibody to mPER2 was first added, and immune complexes were separated from supernatant. The supernatant was transferred to a new tube and subjected to a second IP with anti-mPER1 antibody. Immune complexes and supernatant from both IP reactions were analyzed by WB for mPER1, mPER2, and mPER3. Two arrowheads indicate nonspecific bands that confirm equal loading of total protein. The majority of mPER3 was copurified with mPER1, indicating that mPER1 and not mPER2 is the major binding partner for mPER3.