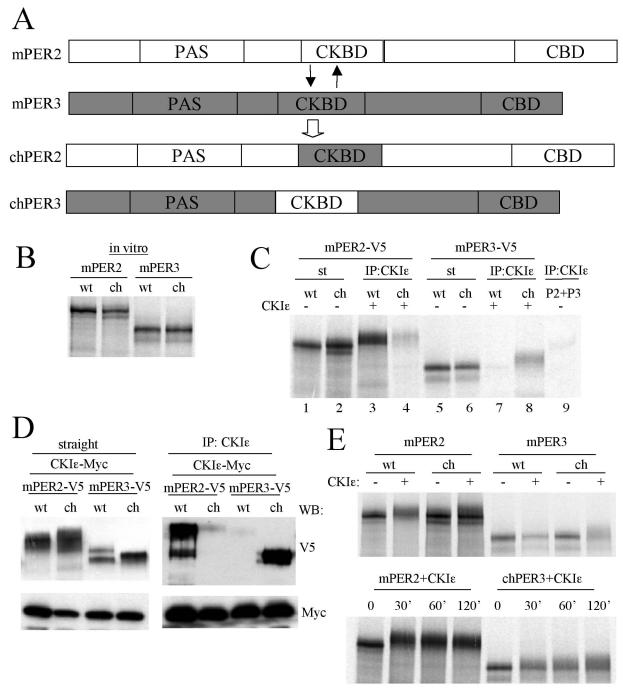
FIG. 8.
CKBD of mPER is important for interaction with CKIɛ and efficient phosphorylation. (A) Diagram of CKBD swapping between mPER2 and mPER3. Amino acids 510 to 709 of mPER3 were exchanged for amino acids 551 to 750 of mPER2. (B) The apparent sizes of chPERs are the same as their wild-type counterparts. mPER2, mPER3, chPER2, and chPER3 were synthesized in vitro in the presence of l-[35S]methionine, and they were resolved by SDS-PAGE. (C) chPER3 binds to CKIɛ more efficiently than mPER3, whereas chPER2 binds to CKIɛ much less efficiently than mPER2. The PERs and CKIɛ were synthesized in vitro in the presence of l-[35S]methionine. Each of the PERs was mixed with CKIɛ at a molar ratio of 1:3. The mixtures were subjected to IP with CKIɛ antibody, and the immune complexes were resolved by SDS-PAGE and visualized by autoradiography. CKIɛ antibody immunoprecipitated >95% of input CKIɛ in all cases (data not shown). When CKIɛ was absent from the mixtures, mPER2 and mPER3 were not significantly immunoprecipitated by anti-CKIɛ antibody (last lane). Slow-migrating forms were visible for copurified PER proteins (lanes 3, 4, and 8) compared to input PERs (lanes 1, 2, and 6). (D) Results similar to those in panel C were obtained with transiently expressed proteins in NIH 3T3 cells. Native and chimeric PERs were coexpressed with CKIɛ in NIH 3T3 cells. The cell lysates were subjected to IP with anti-CKIɛ antibody, and the immune complexes were Western blotted with anti-V5 and Myc antibodies (right panel). The left panel indicates the amounts of mPERs and CKIɛ present in the cell lysates. (E) Phosphorylation of chPER3 is enhanced, but that of chPER2 is attenuated compared to their wild-type counterparts in vitro. In vitro-translated PER proteins were incubated with or without CKIɛ for 60 min. at room temperature (upper panel) or incubated with CKIɛ for the indicated times (lower panel). The mixtures were then resolved by SDS-PAGE and visualized by autoradiography. Representative autoradiograms are shown.