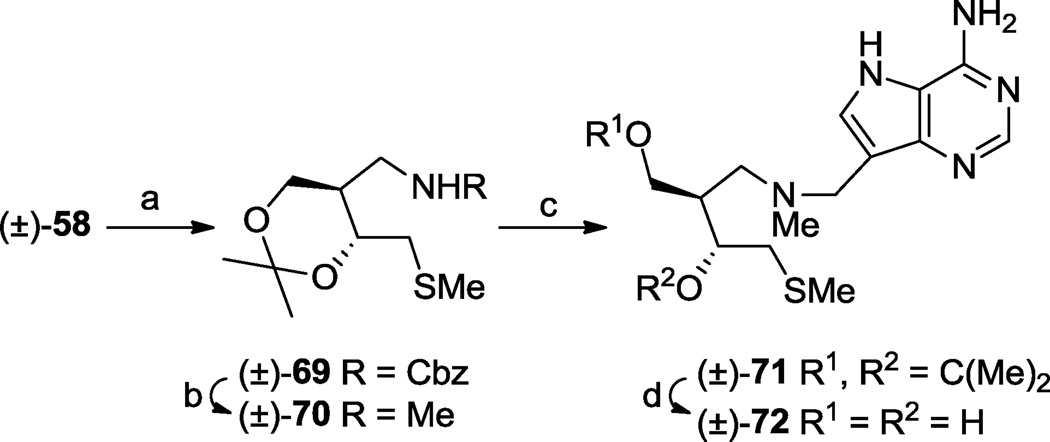
Scheme 10.
One enantiomer series present in the racemic mixtures is drawn to illustrate relative stereochemistry. (a) (i) MsCl, i-Pr2NEt, CH2Cl2, rt 15 min, (ii) NaSMe, DMF, rt, 1 h, 86%; (b) LAH, THF, rt, 18 h, 57%; (c) 9-deazaadenine, CH2O, 1,4-dioxane, H2O, 85 °C, 15 min, 68%; (d) aq. HCl (37%), MeOH, rt, 79%.