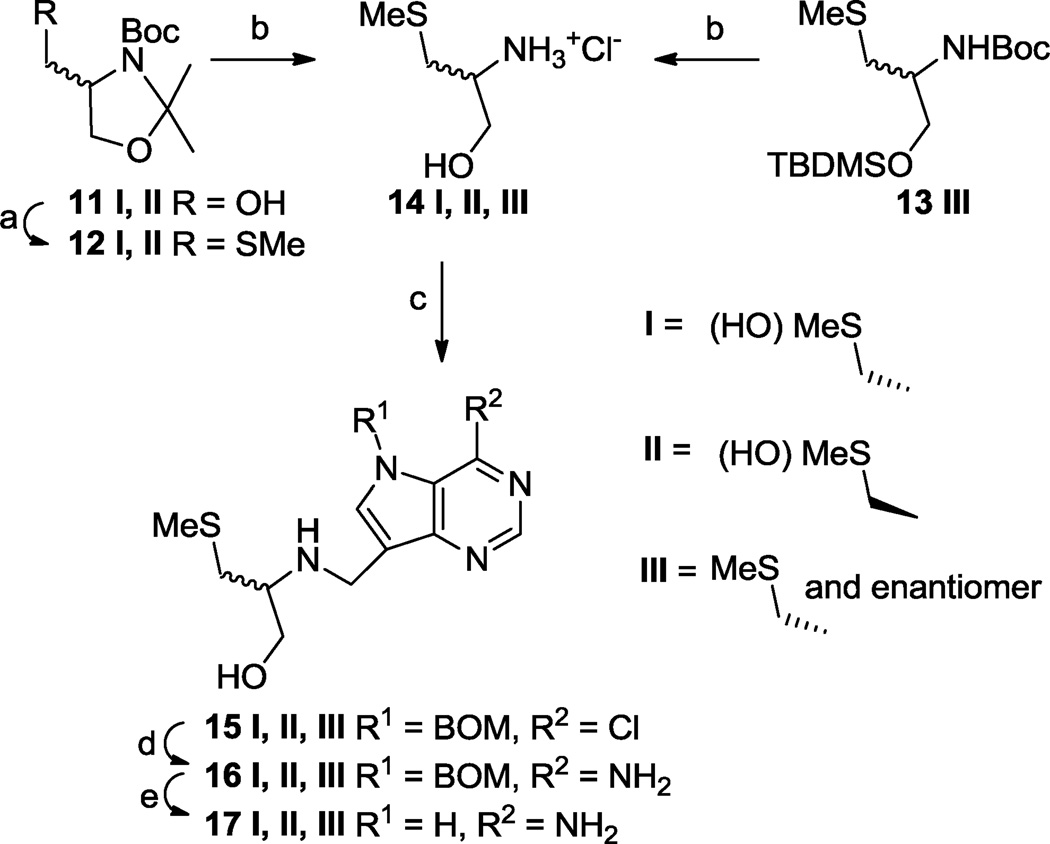
Scheme 2.
(a) (i) MsCl, Et3N, CH2Cl2, 0 °C → rt, 30 min, (ii) NaSMe, DMF, rt, 1 h, 75% (I), 91% (II); (b) aq. HCl (37%), MeOH, 0 °C → rt, 1 h, 100% (I, II and III); (c) 10, 2-picoline-borane complex (for I and II) or NaCNBH3 (for III), MeOH, Et3N (for I and II) or NaHCO3 (for III), rt, 16 h, 67% (I), 78% (II), 71% (III); (d) 7M NH3-MeOH, 135 °C, sealed tube, 24–30 h, 74% (I), 86% (II), 59% (III); (e) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 84% (I), 79% (II), 76% (III).