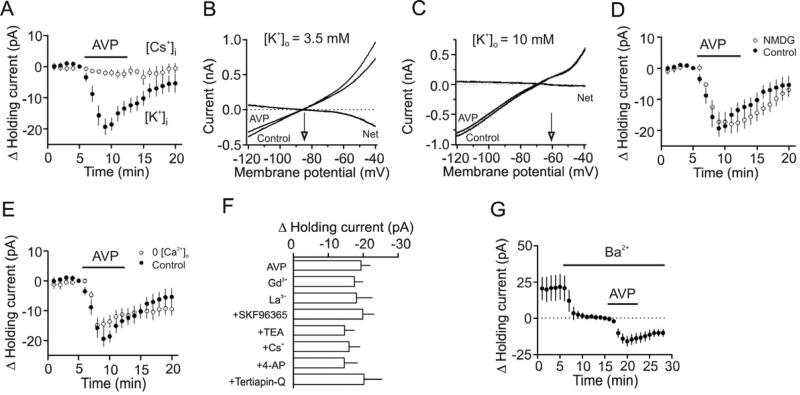
Fig. 4.
AVP-induced depolarization of interneurons is mediated by inhibition of background K+ channels. A, AVP did not induce conspicuous inward HCs when the intracellular solution contained Cs-gluconate. B, Voltage-current relationship recorded by a ramp protocol (from -120 mV to -40 mV, at a speed of 0.07 mV/ms) in the extracellular solution containing 3.5 mM K+ before and during the application of AVP (0.3 μM). Traces in the figure were averaged traces from 5 cells. The AVP-induced net current has a reversal potential at ~-84 mV close to the calculated K+ reversal potential (~-85 mV). C, Voltage-current relationship recorded by the same ramp protocol in the extracellular solution containing 10 mM K+ before and during the application of AVP (0.3 μM). Traces in the figure were averaged traces from 6 cells. The AVP-induced net current has a reversal potential at ~-61 mV close to the calculated K+ reversal potential (~-58.7 mV). D, Replacement of extracellular NaCl with NMDG-Cl failed to change AVP-mediated increases in inward HCs significantly. E, Bath application of AVP (0.3 μM) still induced a comparable inward HC in the extracellular solution containing 0 Ca2+. F, Inclusion of Gd3+ (10 μM), La3+ (10 μM) or SKF96365 (100 μM) in the extracellular solution had no effects on AVP-induced increases in inward HCs. Similarly, inclusion of the classical K+ channel blockers (TEA, Cs+, 4-AP, tertiapin-Q) in the extracellular solution did not significantly alter AVP-mediated increases in inward HCs. G, Bath application of Ba2+ (2 mM) induced an inward HC by itself but did not block AVP-induced increases in inward HCs.