Fig. 5.
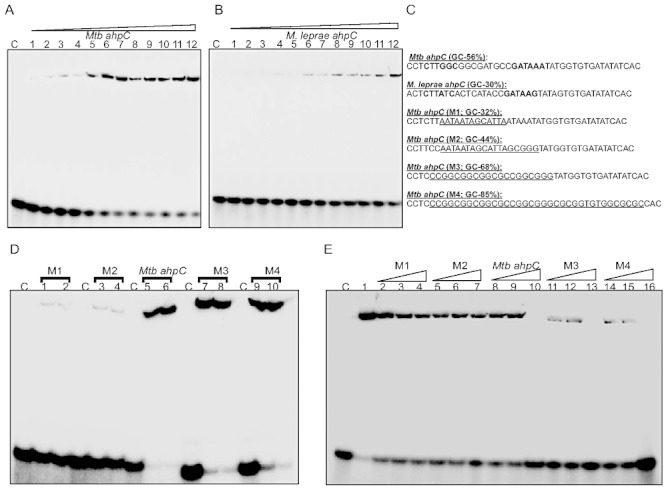
WhiB4 binds to GC-rich DNA. A and B. Concentration-dependent binding of oxidized apo-WhiB4 to a 40 bp γ-32P-labelled DNA fragment derived from (A) Mtb and (B) M. leprae ahpC promoter regions containing the OxyR-binding motif. The concentrations of oxidized apo-WhiB4 were 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, 2.0, 2.25, 2.50, 2.75, 3.00 µM. The Kd for both the fragments was calculated by measuring the intensity of free and protein bound DNA using ImageJ software. Note that apo-WhiB4 binds with higher affinity to the GC-rich Mtb OxyR recognition sequence as compared with the AT-rich M. leprae OxyR recognition sequence within the ahpC promoter. C. DNA fragments containing mutations to modify the GC content of the ahpC promoter region (OxyR binding core motif is shown in bold). The mutated sequences in various DNA fragments (M1–M4) are underlined. These fragments were subjected to EMSA analysis. D. EMSAs were carried out using 0.8 and 1 µM oxidized apo-WhiB4 and 0.2 nM γ-32P-labelled DNA fragments. Note that apo-WhiB4 showed enhanced binding to M3 (68% GC) and M4 (85% GC) as compared with M1 (32% GC) and M2 (44% GC) fragments. E. Competition assay using high and low GC DNA fragments. Lane 1: oxidized apo-WhiB4:ahpC promoter complex. DNA binding was competed using 10-fold (lanes 2, 5, 8, 11 and 14), 20-fold (lanes 3, 6, 9, 12 and 15) and 50-fold (lanes 4, 7, 10, 13 and 16) molar excess of unlabelled DNA fragments as indicated in the figure. C: DNA binding in the absence of WhiB4 in each panel.
