Fig. 6.
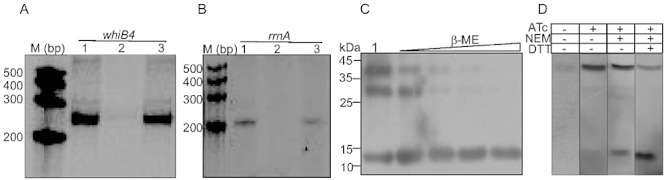
A and B. Effect of WhiB4 on in vitro transcription. Single round transcription assays show that RNAP-σA holoenzyme was proficient in directing transcription from whiB4 (A, lane 1) and rrnA (B, lane 1) promoters. 50 nM of whiB4 and rrnA promoter DNA fragments were pre-incubated with either 2 µM oxidized apo-WhiB4 (A, lane 2; B, lane 2) or reduced apo-WhiB4 (A, lane 3; B, lane 3) and subjected to transcription by RNAP-σA. M: RNA marker (Century™ Marker Template, Ambion). C. Disulphide bond formation induces oligomerization of apo-WhiB4 in vitro. Five micrograms of apo-WhiB4 is either oxidized by atmospheric O2 (lane 1) or reduced by 50 mM, 100 mM, 200 mM and 400 mM β-ME and resolved on a 12% non-reducing SDS-PAGE. Apo-WhiB4 bands were visualized by Western blot analysis using anti-His antibody. The ∼ 14 kDa, ∼ 28 kDa and ∼ 42 kDa bands correspond to the His-tagged apo-WhiB4-monomer, -dimer and -trimer. D. In vivo existence of disulphide-linked oligomers of apo-WhiB4. Aerobically grown Msm WhiB4 FLAG-tag strain was either uninduced or induced with ATc and 30 µg of cell-free extract was analysed by non-reducing Western blot using anti-FLAG antibody. Note that a significant portion of the FLAG-tagged apo-WhiB4 exists as a trimer in ATc induced Msm cells. To minimize the possibility of O2-induced thiol oxidation and subsequent oligomerization of apo-WhiB4 during cell-free extract preparation, Msm cells expressing FLAG-tagged WhiB4 were pretreated with the thiol-alkylating agent NEM. Note the presence of apo-WhiB4 trimer in the NEM-pretreated sample. A significant loss of apo-WhiB4 oligomerization upon DTT reduction further suggests the presence of intermolecular disulphide-linked oligomers in vivo.
